Abstract
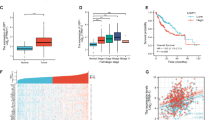
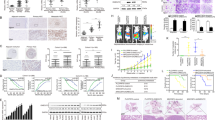
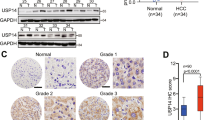
USP11 is a member of the ubiquitin-specific protease family and plays a crucial role in tumor progression in various cancers. However, the precise mechanism by which USP11 promotes EMT and metastasis in hepatocellular carcinoma (HCC) is not fully understood. In this study, we demonstrated that the USP11 expression was dramatically upregulated in HCC tissues and cell lines. Increased USP11 expression was closely associated with tumor number, vascular invasion, and poor prognosis. Functional experiments demonstrated that USP11 markedly promoted metastasis and EMT in HCC via induction of the transcription factor Snail. Mechanistically, USP11 interacted with and deubiquitinated eEF1A1 on Lys439, thereby inhibiting its ubiquitin-mediated degradation. Subsequently, the elevated expression of eEF1A1 resulted in its binding to SP1, which in turn drove the binding of SP1 to its target HGF gene promoter to increase its transcription. This led to an enhanced expression of HGF and the activation of the downstream PI3K/AKT signaling pathway. We demonstrated that USP11 promotes EMT and metastasis in HCC via eEF1A1/SP1/HGF dependent-EMT. Our findings suggest that the USP11/ eEF1A1/SP1/HGF axis contributes to metastasis in HCC, and therefore, could be considered as a potential therapeutic target for the treatment of HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The datasets used and analysed during the current study are available from the corresponding author upon reasonable request.
References
Craig AJ, von Felden J, Garcia-Lezana T, Sarcognato S, Villanueva A (2019). Tumour evolution in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. https://doi.org/10.1038/s41575-41019-40229-41574.
Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450–62.
Deng T, Yan G, Song X, Xie L, Zhou Y, Li J, et al. Deubiquitylation and stabilization of p21 by USP11 is critical for cell-cycle progression and DNA damage responses. Proc Natl Acad Sci USA. 2018;115:4678–83.
Swanson DA, Freund CL, Ploder L, McInnes RR, Valle D. A ubiquitin C-terminal hydrolase gene on the proximal short arm of the X chromosome: implications for X-linked retinal disorders. Hum Mol Genet. 1996;5:533–8.
Lee EW, Seong D, Seo J, Jeong M, Lee HK, Song J. USP11-dependent selective cIAP2 deubiquitylation and stabilization determine sensitivity to Smac mimetics. Cell Death Differ. 2015;22:1463–76.
Ting X, Xia L, Yang J, He L, Si W, Shang Y, et al. USP11 acts as a histone deubiquitinase functioning in chromatin reorganization during DNA repair. Nucleic Acids Res. 2019;47:9721–40.
Xu Z, Li X, Chen J, Zhao J, Wang J, Ji Y, et al. USP11, Deubiquitinating Enzyme, Associated with Neuronal Apoptosis Following Intracerebral Hemorrhage. J Mol Neurosci. 2016;58:16–27.
Yu M, Liu K, Mao Z, Luo J, Gu W, Zhao W. USP11 Is a Negative Regulator to γH2AX Ubiquitylation by RNF8/RNF168. J Biol Chem. 2016;291:959–67.
Wu H-C, Lin Y-C, Liu C-H, Chung H-C, Wang Y-T, Lin Y-W, et al. USP11 regulates PML stability to control Notch-induced malignancy in brain tumours. Nat Commun. 2014;5:3214.
Jacko AM, Nan L, Li S, Tan J, Zhao J, Kass DJ, et al. De-ubiquitinating enzyme, USP11, promotes transforming growth factor β-1 signaling through stabilization of transforming growth factor β receptor II. Cell Death Dis. 2016;7:e2474.
Sun H, Ou B, Zhao S, Liu X, Song L, Liu X, et al. USP11 promotes growth and metastasis of colorectal cancer via PPP1CA-mediated activation of ERK/MAPK signaling pathway. EBioMedicine. 2019;48:236–47.
Wang W, Wang J, Yan H, Zhang K, Liu Y. Upregulation of USP11 promotes epithelial‑to‑mesenchymal transition by deubiquitinating Snail in ovarian cancer. Oncol Rep. 2019;41:1739–48.
Zhang S, Xie C, Li H, Zhang K, Li J, Wang X, et al. Ubiquitin-specific protease 11 serves as a marker of poor prognosis and promotes metastasis in hepatocellular carcinoma. Lab Invest. 2018;98:883–94.
Qiao L, Zhang Q, Sun Z, Liu Q, Wu Z, Hu W, et al. The E2F1/USP11 positive feedback loop promotes hepatocellular carcinoma metastasis and inhibits autophagy by activating ERK/mTOR pathway. Cancer Lett. 2021;514:63–78.
Yang H, Park D, Ryu J, Park T. USP11 degrades KLF4 via its deubiquitinase activity in liver diseases. J Cell Mol Med. 2021;25:6976–87.
Chen S-L, Lu S-X, Liu L-L, Wang C-H, Yang X, Zhang Z-Y, et al. eEF1A1 Overexpression Enhances Tumor Progression and Indicates Poor Prognosis in Hepatocellular Carcinoma. Transl Oncol. 2018;11:125–31.
Bouattour M, Raymond E, Qin S, Cheng A-L, Stammberger U, Locatelli G, et al. Recent developments of c-Met as a therapeutic target in hepatocellular carcinoma. Hepatology. 2018;67:1132–49.
Nieto MA, Huang RY-J, Jackson RA, Thiery JP. EMT: 2016. Cell. 2016;166:21–45.
Santamaria PG, Moreno-Bueno G, Portillo F, Cano A. EMT: Present and future in clinical oncology. Mol Oncol. 2017;11:718–38.
Huang J, Zheng C, Shao J, Chen L, Liu X, Shao J. Overexpression of eEF1A1 regulates G1-phase progression to promote HCC proliferation through the STAT1-cyclin D1 pathway. Biochem Biophys Res Commun. 2017;494:542–9.
Zeng C, Sang Y, Wang F-Y, Zhuang S-M. Opposing roles of C/EBPα and eEF1A1 in Sp1-regulated miR-122 transcription. RNA Biol. 2020;17:202–10.
Jiang JG, Chen Q, Bell A, Zarnegar R. Transcriptional regulation of the hepatocyte growth factor (HGF) gene by the Sp family of transcription factors. Oncogene. 1997;14:3039–49.
Shah P, Qiang L, Yang S, Soltani K, He Y-Y. Regulation of XPC deubiquitination by USP11 in repair of UV-induced DNA damage. Oncotarget. 2017;8:96522–35.
Koiwai K, Maezawa S, Hayano T, Iitsuka M, Koiwai O. BPOZ-2 directly binds to eEF1A1 to promote eEF1A1 ubiquitylation and degradation and prevent translation. Genes Cells. 2008;13:593–607.
Liu X, Chen L, Ge J, Yan C, Huang Z, Hu J, et al. The Ubiquitin-like Protein FAT10 Stabilizes eEF1A1 Expression to Promote Tumor Proliferation in a Complex Manner. Cancer Res. 2016;76:4897–907.
Schuller AP, Green R. Roadblocks and resolutions in eukaryotic translation. Nat Rev Mol Cell Biol. 2018;19:526–41.
Abbas W, Kumar A, Herbein G. The eEF1A Proteins: At the Crossroads of Oncogenesis, Apoptosis, and Viral Infections. Front Oncol. 2015;5:75.
Robichaud N, Sonenberg N, Ruggero D, Schneider RJ. Translational Control in Cancer. Cold Spring Harb Perspect Biol. 2019;11:a032896.
Liu S, Hausmann S, Carlson SM, Fuentes ME, Francis JW, Pillai R, et al. METTL13 Methylation of eEF1A Increases Translational Output to Promote Tumorigenesis. Cell. 2019;176:491–504.
Zhang Y, Xia M, Jin K, Wang S, Wei H, Fan C, et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol Cancer. 2018;17:45.
Yang X, Zhang X-F, Lu X, Jia H-L, Liang L, Dong Q-Z, et al. MicroRNA-26a suppresses angiogenesis in human hepatocellular carcinoma by targeting hepatocyte growth factor-cMet pathway. Hepatology. 2014;59:1874–85.
Horiguchi N, Takayama H, Toyoda M, Otsuka T, Fukusato T, Merlino G, et al. Hepatocyte growth factor promotes hepatocarcinogenesis through c-Met autocrine activation and enhanced angiogenesis in transgenic mice treated with diethylnitrosamine. Oncogene. 2002;21:1791–9.
Vizcaíno C, Mansilla S, Portugal J. Sp1 transcription factor: A long-standing target in cancer chemotherapy. Pharm Ther. 2015;152:111–24.
Acknowledgements
We would like to thank SpecAlly Life Technology Co., Ltd for proteomic expertise.
Funding
This work was supported by The National Natural Science Foundation of China (NO.82003003 to JC, No.81874189 to BZ, NO.81472705 to LJ), State Key Project on Infection Disease of China (No. 2018ZX10723204-003) and Projects of Natural Science Foundation of Hubei Province (Grant No. 2021CFB391 to LJ).
Author information
Authors and Affiliations
Contributions
CXP, ZBX, and JL conceived and designed the project. JC, ND, and DPC performed the experiments. LQM and MJ collected the clinical specimens and data. LHF performed the statistical analysis. CJ and ND drafted the manuscript. ZXW, ZWG, and ZMZ contributed to critical revision of the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, J., Ning, D., Du, P. et al. USP11 potentiates HGF/AKT signaling and drives metastasis in hepatocellular carcinoma. Oncogene 43, 123–135 (2024). https://doi.org/10.1038/s41388-023-02847-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02847-8