Abstract
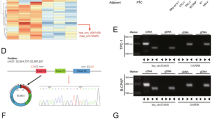
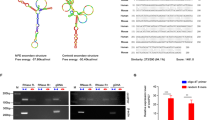
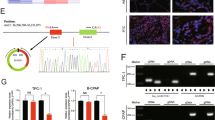
Circular RNAs (circRNAs) play an important role in regulating the development of human cancers through diverse biological functions. However, the exact molecular mechanisms underlying the role of circRNAs in papillary thyroid cancer (PTC) remain largely unknown. Here, we found that hsa_circ_0011385, designated as circular eukaryotic translation initiation factor 3 subunit I (circEIF3I), preferentially localized in the cytoplasm of PTC cells and was more stable than its linear counterpart, EIF3I. Gain- and loss-of-function studies indicated that circEIF3I promoted PTC progression by facilitating cell proliferation, cell cycle, cell migration, and invasion in vitro, as well as PTC cell proliferation in vivo. Mechanistically, circEIF3I interacted with AU-rich element (ARE) RNA-binding factor 1 (AUF1) in the cytoplasm of PTC cells, thus reducing the degradation of Cyclin D1 mRNA and increasing Cyclin D1 protein production, ultimately resulting in PTC progression. Collectively, our results demonstrate the vital role of circEIF3I in PTC progression, supporting its significance as a potential therapeutic target.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data in our study are available from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clinicians. 2021;71:209–49.
Megwalu UC, Moon PK. Thyroid Cancer Incidence and Mortality Trends in the United States: 2000–2018. Thyroid: Off J Am Thyroid Assoc. 2022;32:560–70.
Sugino K, Matsuzu K, Nagahama M, Kitagawa W, Suzuki A, Tomoda C, et al. Impact of Age on Prognosis in Papillary Thyroid Carcinoma: How Should Age be Incorporated into the Treatment Strategy? World J Surgery. 2022;47:674–81.
Vuong HG, Le MK, Hassell L, Kondo T, Kakudo K. The differences in distant metastatic patterns and their corresponding survival between thyroid cancer subtypes. Head Neck. 2022;44:926–32.
Nunes KS, Matos LL, Cavalheiro BG, Magnabosco FF, Tavares MR, Kulcsar MA, et al. Risk factors associated with disease-specific mortality in papillary thyroid cancer patients with distant metastases. Endocrine 2022;75:814–22.
Arianpoor A, Asadi M, Amini E, Ziaeemehr A, Ahmadi Simab S, Zakavi SR. Investigating the prevalence of risk factors of papillary thyroid carcinoma recurrence and disease-free survival after thyroidectomy and central neck dissection in Iranian patients. Acta chirurgica Belgica. 2020;120:173–8.
Chen LL. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol. 2020;21:475–90.
Liu CX, Chen LL. Circular RNAs: Characterization, cellular roles, and applications. Cell 2022;185:2016–34.
Bozzoni I. Widespread occurrence of circular RNA in eukaryotes. Nat Rev Genet. 2021;22:550–1.
Li S, Li X, Xue W, Zhang L, Yang LZ, Cao SM, et al. Screening for functional circular RNAs using the CRISPR-Cas13 system. Nat methods. 2021;18:51–9.
Kristensen LS, Jakobsen T, Hager H, Kjems J. The emerging roles of circRNAs in cancer and oncology. Nat Rev Clin Oncol. 2022;19:188–206.
Cui C, Yang J, Li X, Liu D, Fu L, Wang X. Functions and mechanisms of circular RNAs in cancer radiotherapy and chemotherapy resistance. Mol Cancer. 2020;19:58.
Yao X, Zhang Q. Function and Clinical Significance of Circular RNAs in Thyroid Cancer. Front Mol Biosci. 2022;9:925389.
Peng X, Zhu Y, Lin S, Yu W, Zhang C, Tan L, et al. Circular RNA_0057209 Acts as ceRNA to Inhibit Thyroid Cancer Progression by Promoting the STK4-Mediated Hippo Pathway via Sponging MicroRNA-183. Oxid Med Cell Longev. 2022;2022:9974639.
Zhang D, Tao L, Xu N, Lu X, Wang J, He G, et al. CircRNA circTIAM1 promotes papillary thyroid cancer progression through the miR-646/HNRNPA1 signaling pathway. Cell Death Discov. 2022;8:21.
Chen S, Li K, Guo J, Chen HN, Ming Y, Jin Y, et al. circNEIL3 inhibits tumor metastasis through recruiting the E3 ubiquitin ligase Nedd4L to degrade YBX1. Proc Natl Acad Sci USA. 2023;120:e2215132120.
Ma Q, Yang F, Huang B, Pan X, Li W, Yu T, et al. CircARID1A binds to IGF2BP3 in gastric cancer and promotes cancer proliferation by forming a circARID1A-IGF2BP3-SLC7A5 RNA-protein ternary complex. J Exp Clin Cancer Res: CR. 2022;41:251.
Peng N, Shi L, Zhang Q, Hu Y, Wang N, Ye H. Microarray profiling of circular RNAs in human papillary thyroid carcinoma. PloS one. 2017;12:e0170287.
Guo M, Sun Y, Ding J, Li Y, Yang S, Zhao Y, et al. Circular RNA profiling reveals a potential role of hsa_circ_IPCEF1 in papillary thyroid carcinoma. Mol Med Rep. 2021;24:603.
Wang Y, He H, Li W, Phay J, Shen R, Yu L, et al. MYH9 binds to lncRNA gene PTCSC2 and regulates FOXE1 in the 9q22 thyroid cancer risk locus. Proc Natl Acad Sci USA. 2017;114:474–9.
Li H, Zhou X, Wang G, Hua D, Li S, Xu T, et al. CAR-T Cells Targeting TSHR Demonstrate Safety and Potent Preclinical Activity Against Differentiated Thyroid Cancer. J Clin Endocrinol Metab. 2022;107:1110–26.
Bellucci M, Agostini F, Masin M, Tartaglia GG. Predicting protein associations with long noncoding RNAs. Nat Methods. 2011;8:444–5.
Tsitsipatis D, Grammatikakis I, Driscoll RK, Yang X, Abdelmohsen K, Harris SC, et al. AUF1 ligand circPCNX reduces cell proliferation by competing with p21 mRNA to increase p21 production. Nucl Acids Res. 2021;49:1631–46.
White EJ, Brewer G, Wilson GM. Post-transcriptional control of gene expression by AUF1: mechanisms, physiological targets, and regulation. Biochim Biophys Acta.2013;1829:680–8.
Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46.
Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32:453–61.
Hu L, Zhou M, Xue L, Zhang J. Circular RNA hsa_circ_0011385 contributes to cervical cancer progression through sequestering miR-149-5p and increasing PRDX6 expression. Reprod Biol. 2022;22:100619.
Ni C, Yang S, Ji Y, Duan Y, Yang W, Yang X, et al. Hsa_circ_0011385 knockdown represses cell proliferation in hepatocellular carcinoma. Cell Death Discov. 2021;7:270.
Xia F, Chen Y, Jiang B, Bai N, Li X. Hsa_circ_0011385 accelerates the progression of thyroid cancer by targeting miR-361-3p. Cancer Cell Int. 2020;20:49.
Wang YF, Li MY, Tang YF, Jia M, Liu Z, Li HQ. Circular RNA circEIF3I promotes papillary thyroid carcinoma progression through competitively binding to miR-149 and upregulating KIF2A expression. Am J Cancer Res. 2020;10:1130–9.
Yang C, Wu S, Mou Z, Zhou Q, Dai X, Ou Y, et al. Exosome-derived circTRPS1 promotes malignant phenotype and CD8+ T cell exhaustion in bladder cancer microenvironments. Mol Ther: J Am Soc Gene Ther. 2022;30:1054–70.
Stagsted LVW, O’Leary ET, Ebbesen KK, Hansen TB. The RNA-binding protein SFPQ preserves long-intron splicing and regulates circRNA biogenesis in mammals. eLife. 2021;10:e63088.
Shao M, Hao S, Jiang L, Cai Y, Zhao X, Chen Q, et al. CRIT: Identifying RNA-binding protein regulator in circRNA life cycle via non-negative matrix factorization. Molecular therapy. Nucl Acids. 2022;30:398–406.
Okholm TLH, Sathe S, Park SS, Kamstrup AB, Rasmussen AM, Shankar A, et al. Transcriptome-wide profiles of circular RNA and RNA-binding protein interactions reveal effects on circular RNA biogenesis and cancer pathway expression. Genome Med. 2020;12:112.
Liu Q, Zhu D, Li N, Chen S, Hu L, Yu J, et al. Regulation of LRRK2 mRNA stability by ATIC and its substrate AICAR through ARE-mediated mRNA decay in Parkinson’s disease. The EMBO J. 2023;42:e113410.
Abbadi D, Yang M, Chenette DM, Andrews JJ, Schneider RJ. Muscle development and regeneration controlled by AUF1-mediated stage-specific degradation of fate-determining checkpoint mRNAs. Proc Natl Acad Sci USA. 2019;116:11285–90.
Yoon JH, De S, Srikantan S, Abdelmohsen K, Grammatikakis I, Kim J, et al. PAR-CLIP analysis uncovers AUF1 impact on target RNA fate and genome integrity. Nat Commun. 2014;5:5248.
Zhang Z, Fan W, Gao Q, Han Y, Ma J, Gao W, et al. Hsa_Circ_0000826 inhibits the proliferation of colorectal cancer by targeting AUF1. J Genet Genomics. 2023;50:192–203.
Deng S, Qian L, Liu L, Liu H, Xu Z, Liu Y, et al. Circular RNA ARHGAP5 inhibits cisplatin resistance in cervical squamous cell carcinoma by interacting with AUF1. Cancer Sci. 2023;114:1582–95.
Li X, Tian Z, Jin H, Xu J, Hua X, Yan H, et al. Decreased c-Myc mRNA Stability via the MicroRNA 141-3p/AUF1 Axis Is Crucial for p63α Inhibition of Cyclin D1 Gene Transcription and Bladder Cancer Cell Tumorigenicity. Mol Cellular Biol. 2018;38:e00273–18.
Nardone V, Barbarino M, Angrisani A, Correale P, Pastina P, Cappabianca S, et al. CDK4, CDK6/cyclin-D1 Complex Inhibition and Radiotherapy for Cancer Control: A Role for Autophagy. Int J Mol Sci. 2021;22:8391.
Yang X, Feng Y, Liu Y, Ye X, Ji X, Sun L, et al. Fuzheng Jiedu Xiaoji formulation inhibits hepatocellular carcinoma progression in patients by targeting the AKT/CyclinD1/p21/p27 pathway. Phytomedicine : Int J Phytother Phytopharmacology. 2021;87:153575.
Wang QS, Li F, Liao ZQ, Li K, Yang XL, Lin YY, et al. Low level of Cyclin-D1 correlates with worse prognosis of clear cell renal cell carcinoma patients. Cancer Med. 2019;8:4100–9.
Lee JJ, Au AY, Foukakis T, Barbaro M, Kiss N, Clifton-Bligh R, et al. Array-CGH identifies Cyclin D1 and UBCH10 amplicons in anaplastic thyroid carcinoma. Endocr-Relat cancer. 2008;15:801–15.
Liu J, Tao LL, Yu GY, Chen G, Wang Z, Mei KY, et al. Diagnostic significance of CyclinD1 and D2-40 expression for follicular neoplasm of the thyroid. Pathol, Res Pract. 2022;229:153739.
Khoo ML, Beasley NJ, Ezzat S, Freeman JL, Asa SL. Overexpression of Cyclin D1 and underexpression of p27 predict lymph node metastases in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2002;87:1814–8.
Wu S, Yang J, Xu H, Wang X, Zhang R, Lu W, et al. Circular RNA circGLIS3 promotes bladder cancer proliferation via the miR-1273f/SKP1/Cyclin D1 axis. Cell Biol Toxicol. 2022;38:129–46.
Yang SJ, Wang DD, Zhong SL, Chen WQ, Wang FL, Zhang J, et al. Tumor-derived exosomal circPSMA1 facilitates the tumorigenesis, metastasis, and migration in triple-negative breast cancer (TNBC) through miR-637/Akt1/β-catenin (Cyclin D1) axis. Cell Death Dis. 2021;12:420.
Barrett T, Edgar R. Gene expression omnibus: microarray data storage, submission, retrieval, and analysis. Methods Enzymol. 2006;411:352–69.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 32270590).
Author information
Authors and Affiliations
Contributions
QZ, LC and YHL conceived, designed, and supervised all the experiments. XLY and HYL conducted the experiments and most of the analysis. ZW, QF, YHM, and FTL conducted the clinical data analysis. WYC performed circRNA circularization and RNA EMSA experiments. JZ, YLW, YC and LPX collected the tissue specimens. XLY and HYL wrote and revised the manuscript. LC provided critical discussions and the guidance suggestion to the revised manuscript. All authors read, discussed and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yao, X., Liu, H., Wang, Z. et al. Circular RNA EIF3I promotes papillary thyroid cancer progression by interacting with AUF1 to increase Cyclin D1 production. Oncogene 42, 3206–3218 (2023). https://doi.org/10.1038/s41388-023-02830-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02830-3
This article is cited by
-
Peptidylprolyl isomerase D circular RNA sensitizes breast cancer to trastuzumab through remodeling HER2 N4-acetylcytidine modification
Journal of Applied Genetics (2024)
-
Circ-NUP98 Promotes Lung Adenocarcinoma Development Through Regulating CBX1 by miR-188-3p
Biochemical Genetics (2023)