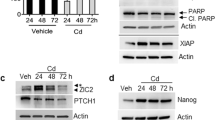
Abstract
Increasing evidence points towards a causal link between exposure to persistent organic pollutants (POPs) with increased incidence and aggressivity of various cancers. Among these POPs, dioxin and PCB-153 are widely found in our environment and represent a significant source of contamination. Dioxin exposure has already been linked to cancer such as non-Hodgkin’s lymphoma, but remains to be more extensively investigated in other cancers. Potential implications of dioxin and PCB-153 in prostate cancer progression spurred us to challenge both ex vivo and in vivo models with low doses of these POPs. We found that dioxin or PCB-153 exposure increased hallmarks of growth and metastasis of prostate cancer cells ex vivo and in grafted NOD-SCID mice. Exposure induced histopathological carcinoma-like patterns in the Ptenpc−/− mice. We identified up-regulation of Acetyl-CoA Acetyltransferase-1 (ACAT1) involved in ketone bodies pathway as a potential target. Mechanistically, genetic inhibition confirmed that ACAT1 mediated dioxin effect on cell migration. Using public prostate cancer datasets, we confirmed the deregulation of ACAT1 and associated gene encoded ketone bodies pathway enzymes such as OXCT1, BDH1 and HMGCL in advanced prostate cancer. To further explore this link between dioxin and ACAT1 deregulation, we analyzed a unique prostate-tumour tissue collection from the USA veterans exposed to agent orange, known to be highly contaminated by dioxin because of industrial production. We found that ACAT1 histoscore is significantly increased in exposed patients. Our studies reveal the implication of dioxin and PCB-153 to induce a prometastatic programme in prostate tumours and identify ACAT1 deregulation as a key event in this process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data are available in the main text or the supplementary materials.
References
International Agency for Research on Cancer Working Group on the Evaluation of Carcinogenic Risks to Humans. 2,3,7,8-tetrachlorodibenzo-para-dioxin, 2,3,4,7,8-pentachlorodibenzofuran, and 3,3′,4,4′,5-pentachlorobiphenyl. In: Chemical agents and related occupations - IARC monographs on the evaluation of carcinogenic risks to humans, 100F. 2012.
International Agency for Research on Cancer Working Group on the Evaluation of Carcinogenic Risks to Humans. Polychlorinated biphenyls and polybrominated biphenyls. IARC monographs on the evaluation of carcinogenic risks to humans, 107; 2016.
Hardell L, Andersson SO, Carlberg M, Bohr L, Van Bavel B, Lindström G, et al. Adipose tissue concentrations of persistent organic pollutants and the risk of prostate cancer. J Occup Environ Med. 2006;48:700–7.
Ali I, Julin B, Glynn A, Högberg J, Berglund M, Johansson JE, et al. Exposure to polychlorinated biphenyls and prostate cancer: Population-based prospective cohort and experimental studies. Carcinogenesis 2016;37:1144–51.
Xu J, Ye Y, Huang F, Chen H, Wu H, Huang J, et al. Association between dioxin and cancer incidence and mortality: a meta-analysis. Sci Rep. 2016;6:38012.
Emeville E, Giusti A, Coumoul X, Thomé JP, Blanchet P, Multigner L. Associations of plasma concentrations of dichlorodiphenyldichloroethylene and polychlorinated biphenyls with prostate cancer: a case–control study in Guadeloupe (French West Indies). Environ Health Perspect. 2015;123:317–23.
Leng L, Chen X, Li CP, Luo XY, Tang NJ. 2,3,7,8-Tetrachlorodibezo-p-dioxin exposure and prostate cancer: A meta-analysis of cohort studies. Public Health. 2014;128:207–13.
Dragan YP, Schrenk D. Animal studies addressing the carcinogenicity of TCDD (or related compounds) with an emphasis on tumour promotion. Food Addit Contam. 2000;17:289–302.
Moore RW, Fritz WA, Schneider AJ, Lin TM, Branam AM, Safe S, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin has both pro-carcinogenic and anti-carcinogenic effects on neuroendocrine prostate carcinoma formation in TRAMP mice. Toxicol Appl Pharmacol. 2016;305:242–9.
Murray IA, Patterson AD, Perdew GH. Aryl hydrocarbon receptor ligands in cancer: Friend and foe. Nat Rev Cancer. 2014;14:801–14.
Kollara A, Brown TJ. Four and a half LIM domain 2 alters the impact of aryl hydrocarbon receptor on androgen receptor transcriptional activity. J Steroid Biochem Mol Biol. 2010;118:51–58.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74.
Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–9.
Koundouros N, Poulogiannis G. Reprogramming of fatty acid metabolism in cancer. Br J Cancer. 2020;122:4–22.
Lynen F, Ochoa S. Enzymes of fatty acid metabolism. BBA - Biochim Biophys Acta. 1953;1000:281–96.
Middleton B, Bartlett K, Romanos A, Vazquez JG, Conde C, Cannon RA, et al. 3-Ketothiolase deficiency. Eur J Pediatr. 1986;144:586–9.
Haapalainen AM, Meriläinen G, Wierenga RK. The thiolase superfamily: condensing enzymes with diverse reaction specificities. Trends Biochem Sci. 2006;31:64–71.
Haapalainen AM, Meriläinen G, Pirilä PL, Kondo N, Fukao T, Wierenga RK. Crystallographic and kinetic studies of human mitochondrial acetoacetyl-CoA thiolase: The importance of potassium and chloride ions for its structure and function. Biochemistry. 2007;46:4305–21.
Fan J, Shan C, Kang HB, Elf S, Xie J, Tucker M, et al. Tyr phosphorylation of PDP1 toggles recruitment between ACAT1 and SIRT3 to regulate the pyruvate dehydrogenase complex. Mol Cell. 2014;53:534–48.
Fan J, Lin R, Xia S, Chen D, Elf SE, Liu S, et al. Tetrameric acetyl-CoA acetyltransferase 1 is important for tumor growth. Mol Cell. 2016;64:859–74.
Saraon P, Cretu D, Musrap N, Karagiannis GS, Batruch I, Drabovich AP, et al. Quantitative proteomics reveals that enzymes of the ketogenic pathway are associated with prostate cancer progression. Mol Cell Proteom. 2013;12:1589–601.
Saraon P, Trudel D, Kron K, Dmitromanolakis A, Trachtenberg J, Bapat B, et al. Evaluation and prognostic significance of ACAT1 as a marker of prostate cancer progression. Prostate. 2014;74:372–80.
Vezina CM, Lin TM, Peterson RE. AHR signaling in prostate growth, morphogenesis, and disease. Biochem. Pharmacol. 2009;77:566–76.
Gupta C. Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals. Proc Soc Exp Biol Med. 2000;224:61–68.
Drost J, Karthaus WR, Gao D, Driehuis E, Sawyers CL, Chen Y, et al. Organoid culture systems for prostate epithelial and cancer tissue. Nat Protoc. 2016;11:347–58.
Karthaus WR, Iaquinta PJ, Drost J, Gracanin A, Van Boxtel R, Wongvipat J, et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 2014;159:163–75.
Lee HM, Hwang KA, Choi KC. Diverse pathways of epithelial mesenchymal transition related with cancer progression and metastasis and potential effects of endocrine disrupting chemicals on epithelial mesenchymal transition process. Mol Cell Endocrinol. 2017;457:103–13.
Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;3:209–21.
Fletcher N, Wahlström D, Lundberg R, Nilsson CB, Nilsson KC, Stockling K, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) alters the mRNA expression of critical genes associated with cholesterol metabolism, bile acid biosynthesis, and bile transport in rat liver: a microarray study. Toxicol Appl Pharmacol. 2005;207:1–24.
Cholico GN, Fling RR, Zacharewski NA, Fader KA, Nault R, Zacharewski TR. Thioesterase induction by 2,3,7,8-tetrachlorodibenzo-p-dioxin results in a futile cycle that inhibits hepatic β-oxidation. Sci Rep. 2021;11:15689.
Labanca E, Bizzotto J, Sanchis P, Anselmino N, Yang J, Shepherd PDA, et al. Prostate cancer castrate resistant progression usage of non-canonical androgen receptor signaling and ketone body fuel. Oncogene. 2021;40:6284–98.
Gearing LJ, Cumming HE, Chapman R, Finkel AM, Woodhouse IB, Luu K, et al. CiIIder: A tool for predicting and analysing transcription factor binding sites. PLoS ONE. 2019;14:e0215495.
Li S, Pei X, Zhang W, Xie HQ, Zhao B. Functional analysis of the dioxin response elements (DREs) of the murine CYP1A1 gene promoter: beyond the core DRE sequence. Int J Mol Sci. 2014;15:6475–87.
Dere E, Lo R, Celius T, Matthews J, Zacharewski TR. Integration of genome-wide computation DRE search, AhR ChIP-chip and gene expression analyses of TCDD-elicited responses in the mouse liver. BMC Genomics. 2011;12:365.
Poland A, Palen D, Glover E. Tumour promotion by TCDD in skin of HRS/J hairless mice. Nature. 1982;300:271–3.
Knerr S, Schrenk D. Carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models. Mol Nutr Food Res. 2006;50:897–907.
Wang L, Kumar M, Deng Q, Wang X, Liu M, Gong Z, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces peripheral blood abnormalities and plasma cell neoplasms resembling multiple myeloma in mice. Cancer Lett. 2019;440-441:135–44.
Prins GS, Hu WY, Shi GB, Hu DP, Majumdar S, Li G, et al. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology. 2014;155:805–17.
Ghotbaddini M, Powell JB. The AhR ligand, TCDD, regulates androgen receptor activity differently in androgen-sensitive versus castration-resistant human prostate cancer cells. Int J Environ Res Public Health. 2015;12:7506–18.
Peters AK, Leonards PE, Zhao B, Bergman Å, Denison MS, Van, et al. Determination of in vitro relative potency (REP) values for mono-ortho polychlorinated biphenyls after purification with active charcoal. Toxicol Lett. 2006;12:7506–18.
Kopec AK, Burgoon LD, Ibrahim-Aibo D, Mets BD, Tashiro C, Potter D, et al. PCB153-elicited hepatic responses in the immature, ovariectomized C57BL/6 mice: comparative toxicogenomic effects of dioxin and non-dioxin-like ligands. Toxicol Appl Pharmacol. 2010;243:359–71.
Villano CM, Murphy KA, Akintobi A, White LA. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces matrix metalloproteinase (MMP) expression and invasion in A2058 melanoma cells. Toxicol Appl Pharmacol. 2006;210:212–24.
Diry M, Tomkiewicz C, Koehle C, Coumoul X, Bock KW, Barouki R, et al. Activation of the dioxin/aryl hydrocarbon receptor (AhR) modulates cell plasticity through a JNK-dependent mechanism. Oncogene. 2006;25:5570–4.
Seifert A, Rau S, Küllertz G, Fischer B, Santos AN. TCDD induces cell migration via NFATc1/ATX-signaling in MCF-7 cells. Toxicol Lett. 2009;184:26–32.
Bui LC, Tomkiewicz C, Chevallier A, Pierre S, Bats AS, Mota S, et al. Nedd9/Hef1/Cas-L mediates the effects of environmental pollutants on cell migration and plasticity. Oncogene. 2009;28:3642–51.
Gao Z, Bu Y, Liu X, Wang X, Zhang G, Wang E, et al. TCDD promoted EMT of hFPECs via AhR, which involved the activation of EGFR/ERK signaling. Toxicol Appl Pharmacol. 2016;298:48–55.
Haque M, Francis J, Sehgal I. Aryl hydrocarbon exposure induces expression of MMP-9 in human prostate cancer cell lines. Cancer Lett. 2005;225:159–66.
Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, et al. Metabolism disrupting chemicals and metabolic disorders. Reprod Toxicol. 2017;68:3–33.
Shan Q, Li H, Chen N, Qu F, Guo J. Understanding the multiple effects of PCBS on lipid metabolism. Diabetes Metab Syndr Obes. 2020;13:3691–702.
Lakshman MR, Ghosh P, Chirtel SJ. Mechanism of action of 2,3,7,8-tetrachlorodibenzo-p-dioxin on intermediary metabolism in the rat. J Pharmacol Exp Ther. 1991;258:317–9.
Diani-Moore S, Pedro TM, Rifkind AB. Organ-specific effects on glycolysis by the dioxin-activated aryl hydrocarbon receptor. PLoS ONE. 2020;15:e0243842.
Ambolet-Camoit A, Ottolenghi C, Leblanc A, Kim MJ, Letourneur F, Jacques S, et al. Two persistent organic pollutants which act through different xenosensors (alpha-endosulfan and 2,3,7,8 tetrachlorodibenzo-p-dioxin) interact in a mixture and downregulate multiple genes involved in human hepatocyte lipid and glucose metabolism. Biochimie. 2015;116:79–91.
Zhang Y, Song L, Li Z. Polychlorinated biphenyls promote cell survival through pyruvate kinase M2-dependent glycolysis in HeLa cells. Toxicol Mech Methods. 2019;29:428–37.
Martinez-Outschoorn UE, Lin Z, Whitaker-Menezes D, Howell A, Sotgia F, Lisanti MP. Ketone body utilization drives tumor growth and metastasis. Cell Cycle. 2012;11:3964–71.
Garcia-Bermudez J, Birsoy K. Drugging ACAT1 for cancer therapy. Mol Cell. 2016;64:856–7.
Mao T, Qin F, Zhang M, Li J, Li J, Lai M. Elevated serum β-hydroxybutyrate, a circulating ketone metabolite, accelerates colorectal cancer proliferation and metastasis via ACAT1. Oncogene. 2023;42:1889–99.
Zhang G, Huang R, Zhao H, Xia Y, Huang H, Qian M, et al. ACAT1-mediated METTL3 acetylation inhibits cell migration and invasion in triple negative breast cancer. Genes Immun. 2023;24:99–107.
Guan J, Jiang X, Guo Y, Zhao W, Li J, Li Y, et al. Autophagy inhibition and reactive oxygen species elimination by acetyl-CoA acetyltransferase 1 through fused in sarcoma protein to promote prostate cancer. BMC Cancer. 2022;22:1313.
Goudarzi A. The recent insights into the function of ACAT1: A possible anti-cancer therapeutic target. Life Sci. 2019;232:116592.
Nault R, Fader KA, Lydic TA, Zacharewski TR. Lipidomic evaluation of aryl hydrocarbon receptor-mediated hepatic steatosis in male and female mice elicited by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Chem Res Toxicol. 2017;30:1060–75.
Koual M, Cano-Sancho G, Bats AS, Tomkiewicz C, Kaddouch-Amar Y, Douay-Hauser N, et al. Associations between persistent organic pollutants and risk of breast cancer metastasis. Environ Int. 2019;132:105028.
Franken NAP, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–9.
Wu X, Wu J, Huang J, Powell WC, Zhang JF, Matusik RJ, et al. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–69.
Lesche R, Groszer M, Gao J, Wang Y, Messing A, Sun H, et al. Cre/loxP-mediated inactivation of the murine Pten tumor suppressor gene. Genesis. 2002;32:148–9.
Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32:435–43.
Van den Berg M, Birnbaum LS, Denison M, De Vito M, Farland W, Feeley M, et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93:223–41.
Suttie A, Nyska A, Haseman JK, Moser GJ, Hackett TR, Goldsworthy TL. A grading scheme for the assessment of proliferative lesions of the mouse prostate in the TRAMP model. Toxicol Pathol. 2003;31:31–38.
Havens AM, Pedersen EA, Shiozawa Y, Ying C, Jung Y, Sun Y, et al. An in vivo mouse model for human prostate cancer metastasis. Neoplasia. 2008;10:371–80.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8.
Lee E, Choi J, Jo Y, Kim JY, Jang YJ, Lee HM, et al. ACT-PRESTO: Rapid and consistent tissue clearing and labeling method for 3-dimensional (3D) imaging. Sci Rep. 2016;11:18631.
Acknowledgements
The authors thank Sandrine Plantade, Philippe Mazuel and Khirredine Ouchen for mouse care, Anip@th technical staff (iGReD, Clermont-Ferrand) for histological analyses, SC3 and CLIC platforms (iGReD, Clermont-Ferrand) for cell manipulations and imaging, Bioinformatic platform (iGReD, Clermont-Ferrand) for computational analysis, Jean-Paul Saru for western blot analyses. We thank Dr. Arun Sreekumar and Dr. Jie Gohlke (Baylor College of Medecine, Houston) for help and fruitful discussions in the preparation/interpretation of TMA analysis. We warmly thank Kathleen Gates for careful editing of the manuscript. Part of this study was supported by Région Auvergne Rhône Alpes, Fond Européen de Développement Régional (FEDER), Plan National de Recherche sur les Perturbateurs Endocriniens (13-MRES-PNRPE-1-CVS043), Plan-Cancer 2014-2019 for Jean Marc Lobaccaro and Silvère Baron, and Ligue contre le Cancer Rhône Alpes Auvergne et Saône et Loire for Cyrille de Joussineau. JB is a postdoc funded by Plan-Cancer 2014-2019. Prostate Cancer Foundation - VALOR Challenge grant provided support for MI and JJ in the acquisition/prep of PCa tissues. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
Author information
Authors and Affiliations
Contributions
Acquisition of the data: Jb, SB, JML, SD, EB, JPS, ADH. TMA conception and analysis: MI, JJ, MPV, LG, CDS, FPL, MK. Microscopic and confocal imaging: JB, CDS, MV, CdJ. AHR ChIPseq analysis: JB, YR, JM. Conception and design of the manuscript: JB, JML, SB. Analysis and interpretation of the data: JB, JML, SB. Drafting of the manuscript: JB, JML, SB. Critically revising the manuscript: JB, JML, SB, AT, FD, AK, SC, FB.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Buñay, J., Kossai, M., Damon-Soubeyrant, C. et al. Persistent organic pollutants promote aggressiveness in prostate cancer. Oncogene 42, 2854–2867 (2023). https://doi.org/10.1038/s41388-023-02788-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02788-2