Abstract
Prostate cancer is the most common cancer in men and it is estimated that over 350,000 men worldwide die of prostate cancer every year. There remains an unmet clinical need to improve how clinically significant prostate cancer is diagnosed and develop new treatments for advanced disease. Aberrant glycosylation is a hallmark of cancer implicated in tumour growth, metastasis, and immune evasion. One of the key drivers of aberrant glycosylation is the dysregulated expression of glycosylation enzymes within the cancer cell. Here, we demonstrate using multiple independent clinical cohorts that the glycosyltransferase enzyme GALNT7 is upregulated in prostate cancer tissue. We show GALNT7 can identify men with prostate cancer, using urine and blood samples, with improved diagnostic accuracy than serum PSA alone. We also show that GALNT7 levels remain high in progression to castrate-resistant disease, and using in vitro and in vivo models, reveal that GALNT7 promotes prostate tumour growth. Mechanistically, GALNT7 can modify O-glycosylation in prostate cancer cells and correlates with cell cycle and immune signalling pathways. Our study provides a new biomarker to aid the diagnosis of clinically significant disease and cements GALNT7-mediated O-glycosylation as an important driver of prostate cancer progression.
Similar content being viewed by others
Introduction
Prostate cancer is the most common cancer in men and a major cause of cancer-related deaths [1]. The androgen receptor (AR) plays an essential role in the normal growth and development of the prostate gland, as well as in carcinogenesis [2]. The first-line treatments for advanced prostate cancer include androgen deprivation therapy (ADT), but unfortunately most tumours progress to an aggressive state for which ADT becomes ineffective, known as castration-resistant prostate cancer (CRPC). Numerous second generation agents targeting the androgen receptor signalling axis, such as abiraterone [3] and enzalutamide [4] are available for CRPC, however nearly all affected men will also develop resistance to these agents [5]. It is estimated that more than 350,000 men die of prostate cancer yearly [1], and new therapies for advanced disease are urgently needed.
Serum prostate specific antigen (PSA) measurements are often used to aid prostate cancer diagnosis, but this has poor specificity leading to the overtreatment of non-lethal disease [6, 7]. The introduction of multiparametric magnetic resonance imaging (mpMRI) has improved risk stratification for men with suspected prostate cancer [8], but there remains an unmet clinical need to identify new diagnostic and prognostic biomarkers and attention is now turning to minimally invasive urine or blood based tests [9]. The ideal liquid biopsy test would decrease over-diagnosis, allow low risk patients to avoid biopsy, increase the early diagnosis of clinically significant prostate cancer, and allow for accurate monitoring of response to therapy.
Glycosylation is the most common, complex, and dynamic post-translational modification of both membrane bound and secreted proteins [10]. Glycans are fundamental to many biological processes and hence the glycocalyx, a heavily glycosylated extramembrane compartment, is present on every mammalian cell [11]. In cancer, the size of the tumour cell glycocalyx increases with malignant transformation, and this change alters all aspects of disease progression [12,13,14]. Aberrant glycosylation is a hallmark of cancer cells, and cancer-associated glycans have been detected in virtually every cancer type. Glycans hold huge potential for the development of new diagnostic and therapeutic targets and are predicted to be at the forefront of translational developments. Glycan structures change dramatically in prostate cancer, and are linked to a malignant phenotype [15]. Glycan alterations in prostate cancer include changes to PSA glycosylation, tumour hypersialylation, truncated O-glycans and increased core fucosylation, but the mechanisms driving these changes are poorly understood [15]. One of the key drivers of aberrant glycosylation in cancer is the dysregulated expression of glycosylation enzymes within the cancer cell [16].
Previously, we identified glycosylation as an androgen-regulated process in prostate cancer and further defined a set of glycosylation enzymes that are upregulated in tumour tissue [17,18,19,20]. Among these enzymes, here we have focused on GALNT7, a glycosyltransferase transferring an N-acetylgalactosamine (GalNAc) moiety to Ser/Thr side chains on proteins initiating the so-called O-linked glycan biosynthesis [21]. GALNT7 belongs to a large family of 20 GALNT isoenzymes and is characterized for exclusively glycosylating proteins/peptides that already carry GalNAc moieties added by other GALNT-isoenzymes [22]. Recently, GALNT7 was identified as a single gene effector of cell surface glycosylation and glycocalyx height [23], suggesting that GALNT7 mediated O-glycosylation might act at the interface of cellular signalling processes in prostate cancer and play an important role in disease progression.
Here, we analyse multiple independent cohorts of patient tissue samples and find upregulation of GALNT7 is a feature of prostate cancer cells. Furthermore, we show GALNT7 is found in diagnostically relevant levels in the urine and blood of men with prostate cancer. Our findings reveal urine GALNT7 can identify men with prostate cancer with improved accuracy compared to serum PSA levels, and show combining GALNT7 with serum PSA further improves diagnostic performance. Using lectin/antibody immunoassays, chemical tools and O-glycoproteomics, we show GALNT7 modifies O-glycoproteins in prostate cancer cells, and using both in vitro and in vivo studies, we further show that GALNT7 drives prostate tumour growth and correlates with cell cycle and immune signalling pathways. Our findings identify GALNT7 as an important driver of prostate cancer progression, and highlight new opportunities to exploit aberrant O-glycosylation to improve diagnosis and treatment.
Results
Upregulation of GALNT7 is a feature of prostate cancer
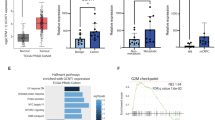
We previously reported preliminary findings suggesting that GALNT7 gene levels are upregulated in clinical prostate cancer tissue, compared to normal or benign prostate tissue [17]. Here, we monitor GALNT7 at both the gene and protein level in multiple independent patient cohorts (comprising >2000 patient samples) and establish upregulation of GALNT7 as a feature of prostate cancer. Analysis of RNA sequencing data from The Cancer Genome Atlas Prostate Adenocarcinoma (TCGA PRAD) cohort [24] reveals GALNT7 gene expression levels are 1.9 fold increased in prostate tumours relative to normal prostate tissue (n = 549, p < 0.001) (Fig. 1A). Using quantitative PCR, we further show upregulation of the GALNT7 gene in prostate cancer tissue in two additional primary patient cohorts (Fig. 1B, C). To test whether GALNT7 is also upregulated at the protein level in prostate tumours, we used immunohistochemistry to monitor GALNT7 protein expression in three independent tissue microarrays (TMA). Staining a tissue microarray containing 25 samples from patients with benign disease (benign prostate hyperplasia, BPH) and 122 samples from patients with prostate cancer [25] showed that GALNT7 protein is expressed at 2.4 fold higher levels in prostate cancer tissue relative to benign tissue (p < 0.001) (Fig. 1D). Similarly, analysis of a TMA containing 17 normal prostate tissue samples and 79 samples of prostate tumour tissue showed GALNT7 is 2.3 fold upregulated in prostate cancer (p = 0.0124) (Supplementary Figure 1). Furthermore, staining of a TMA containing matched normal and prostate cancer tissue from 200 patients [26] showed GALNT7 levels were 1.8 fold higher in prostate cancer tissue compared to matched normal tissue from the same patient (p < 0.001) (Fig. 1E). We confirmed specificity of our GALNT7 immunohistochemistry via pre-incubation with a blocking peptide and by detection of protein depletion and overexpression by immunohistochemistry in Formalin Fixed Paraffin Embedded (FFPE) cell pellets (Supplementary Fig. 2). Taken together, our data identifies upregulation of GALNT7 as a feature of prostate tumour tissue.
Analysis of GALNT7 gene and protein levels in clinical prostate tissue. A GALNT7 mRNA levels in the TCGA PRAD cohort [24] are significantly higher in prostate cancer tissue relative to normal prostate tissue (n = 549, unpaired t test, p = <0.001). B Real-time PCR analysis of GALNT7 in RNA samples extracted from FFPE prostate tissue. GALNT7 is significantly upregulated in cancer relative to benign tissue (n = 12, unpaired t test, p = 0.0174). C Real-time PCR analysis of GALNT7 mRNA in matched normal and prostate tumour samples. GALNT7 is upregulated in tumour tissue relative to matched normal tissue from the same patient (n = 20, paired t test, p = 0.0357). D Immunohistochemistry analysis of GALNT7 protein in a previously published prostate cancer tissue microarray (TMA) [25]. Each section was scored using the Histoscore method [77, 78]. GALNT7 is upregulated in prostate cancer tissue relative to benign prostate hyperplasia tissue (BPH) (n = 147, unpaired t test, p < 0.001). Scale bar is 100 µm. E Immunohistochemistry analysis of GALNT7 protein in a previously published TMA containing matched tumour and normal tissue from the same patient [79]. GALNT7 protein is significantly increased in prostate tumour tissue relative to matched normal tissue from the same patient (n = 200, paired t test, p < 0.001). Scale bar is 100 µm.
GALNT7 is upregulated in urine and blood samples from men with prostate cancer
We next assessed the suitability of GALNT7 as a diagnostic biomarker in biological fluids, compared to the gold standard marker PSA. We noted that the localisation of both proteins in prostate cancer tissue was similar, which was unexpected because GALNT7 is a Golgi-resident enzyme in healthy cells while PSA is secreted into blood. Based on this, and because shedding of glycosylatransferases has been previously reported [27,28,29,30], we hypothesised that like PSA, GALNT7 may also be secreted into the urine and blood of men with prostate cancer. Using pre-validated sandwich ELISA assays (Supplementary Figure 3A), we tested GALNT7 protein levels in urine and blood samples from men with suspected prostate cancer (our power calculations showed testing at least 20 samples would give us a 90% chance of detecting any significant changes, p < 0.05). First, we monitored GALNT7 in matched urine and plasma samples from 27 men with suspected prostate cancer. GALNT7 levels were 7.7 fold higher in urine and 2.1 fold higher in plasma samples from men later diagnosed with prostate cancer compared to men with benign disease or a ‘no cancer’ diagnosis (Fig. 2A, B). Next, we monitored GALNT7 plasma levels in 305 men diagnosed with either benign disease or prostate cancer. GALNT7 protein levels were 2.2 fold higher in men with prostate cancer compared to men diagnosed with benign disease (p < 0.0001) (Fig. 2C). GALNT7 urine levels were also monitored in 180 men with suspected prostate cancer taking part in the INNOVATE clinical trial [31]. Here, urine GALNT7 was 2.5 fold higher in men diagnosed with prostate cancer (compared to men given a ‘no cancer’ diagnosis) (Fig. 2D). Urine GALNT7 had slightly improved accuracy over serum PSA at identifying men with prostate cancer within the same cohort (serum PSA: AUC 0.69, urine GALNT7: AUC 0.73), whereas combining PSA and GALNT7 further increased diagnostic accuracy (PSA + GALNT7: AUC 0.76) (Fig. 2E and Supplementary Fig. 3B).
Detection of GALNT7 in patient urine and blood samples from 3 patient cohorts using pre-validated sandwich ELISA assays. A, B GALNT7 protein levels detected by sandwich ELISA assays in urine and matched plasma samples from men with suspected prostate cancer. Compared to men with benign disease, GALNT7 urine levels are higher in men with prostate cancer (n = 27, unpaired t test, p = 0.082) (AUC 0.78, sensitivity 87%, specificity 58%). Additionally, GALNT7 plasma levels are significantly higher in men with prostate cancer (n = 27, unpaired t test, p = 0.0172)(AUC 0.79, sensitivity 93%, specificity 42%). C GALNT7 levels are significantly higher in plasma samples from men with prostate cancer compared to men with benign disease (n = 305, unpaired t test, p < 0.0001)(AUC 0.83, sensitivity 85%, specificity 65%). D GALNT7 urine levels were monitored via sandwich ELISA assays in 180 men with suspected prostate cancer taking part in the INNOVATE clinical trial [31]. GALNT7 urine levels are significantly higher in men who were diagnosed with prostate cancer, compared to men who received a ‘no cancer’ diagnosis (n = 180, unpaired t test, p < 0.0001). GALNT7 urine level was more accurate than serum PSA at identifying men with prostate cancer (urine GALNT7 AUC: 0.73, sensitivity 85%, specificity 41%)(serum PSA AUC: sensitivity 85%, specificity 38%). E For the 180 men with suspected prostate cancer taking part in the INNOVATE clinical trial, the combination of serum PSA and urine GALNT7 had the greatest diagnostic power to identify those with prostate cancer (AUC 0.76, sensitivity 85%, specificity 58%). F For men taking part in the INNOVATE study that received mpMRI scored Likert 3, GALNT7 levels were significantly higher in men with prostate cancer (n = 59, unpaired t test, p = 0.0001)(AUC 0.78).
The five-point Likert scaling on mpMRI can be used to predict clinically significant prostate cancer (Likert score 1 or 2 indicates the patient is unlikely to have prostate cancer. Likert score 4 or 5 indicates the patient is likely to have prostate cancer that needs to be treated, and with Likert score 3 it is difficult to tell from the scan if prostate cancer is present or not). Hence, for men with an equivocal mpMRI score of Likert 3 (where it isn’t possible to tell from the scan whether the patient has prostate cancer) there is an unmet clinical need for novel biomarkers to aid in risk assessment [32]. To test whether GALNT7 could improve the stratification of patients undergoing mpMRI, we evaluated GALNT7 levels in men from the INNOVATE study with mpMRI scored Likert 3 that later underwent prostate biopsy (n = 59). GALNT7 was 2.6 fold higher in Likert 3 patients that received a prostate cancer diagnosis compared to men given a ‘no cancer’ diagnosis (p = 0.0001), and GALNT7 was slightly more accurate than serum PSA at identifying men with prostate cancer (serum PSA: AUC = 0.69, urine GALNT7: AUC = 0.78) (Fig. 2F). This finding suggests urine GALNT7 levels could aid in risk assessment for patients with mpMRI scored Likert 3 and add value to the clinical care pathway. GALNT7 was also detected in urine from men with metastatic CRPC (Supplementary Fig. 3C). Taken together, our findings show that GALNT7 is upregulated in the urine and blood of men with prostate cancer and could aid in diagnosis.
GALNT7 levels remain high in castrate resistant prostate cancer
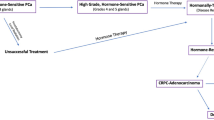
Androgen hormones play a key role in the progression and treatment of prostate cancer and ADT is a first-line treatment used to control cancer growth in cases of metastatic prostate cancer [2]. We previously showed that expression of GALNT7 is controlled by the AR and that GALNT7 is upregulated in prostate cancer cells in response to androgen stimulation [17]. Here, experimental analyses of gene expression data in The Cancer Genome Atlas Prostate Adenocarcinoma (TCGA PRAD) [24] and the metastatic CRPC Stand Up to Cancer/Prostate Cancer Foundation (SU2C/PCF) [33] cohorts show a significant correlation between the expression of AR and GALNT7 in both clinical prostate cancer datasets (Supplementary Fig. 4A, B). Further experimental analyses reveal GALNT7 mRNA is downregulated in prostate tissue following androgen depletion (Supplementary Fig. 4C, D) [34,35,36]. Based on these findings, we hypothesised that GALNT7 protein levels in prostate tissue will decrease in patients undergoing ADT. To test this, we monitored GALNT7 protein levels in a TMA containing untreated prostate tissue and tissue samples taken 3-12 months after ADT (n = 162) [26]. Consistent with GALNT7 being controlled by androgens, our findings show GALNT7 protein levels are 1.5 fold lower in prostate tissue following ADT, compared to untreated tissue (p = 0.001) (Fig. 3A). Next, to gain insight into how GALNT7 is expressed during prostate cancer progression, we analysed GALNT7 in a TMA with hormone sensitive and CRPC patients (n = 125). Here, GALNT7 levels were similar in CRPC compared to hormone sensitive disease, which would be consistent with AR reactivation in castrate resistant disease (Fig. 3B). We then set out to test if serum levels of GALNT7 might also change upon ADT. In matched serum samples from ten men taken before and after hormone ablation therapy, serum GALNT7 was significantly reduced following ADT (p = 0.0039) (Fig. 3C). Furthermore, GALNT7 levels were significantly higher in sera from patients with metastatic CRPC relative to patients with hormone sensitive disease (p < 0.001) (Fig. 3D). Finally, analysis of GALNT7 gene expression in the SU2C/PCF mCRPC tissue cohort [33] identified a significant correlation between GALNT7 and androgen receptor signalling in metastatic CRPC patient samples (n = 163, FDR q value <0.001)(Fig. 3E and Supplementary Table 1). Of particular interest, significant correlations were observed between GALNT7 and CAMKK2, TMPRSS2, CCND1 and FKBP5. These results indicate that GALNT7 levels directly correlate with AR activity in prostate cancer and suggest GALNT7 levels may mark those developing relapse to castrate resistant disease.
Analysis of GALNT7 protein levels across two prostate cancer tissue microarrays (TMAs) using immunohistochemistry and in serum samples using sandwich ELISA assays. A Immunohistrochemistry analysis of GALNT7 levels in a previously published TMA [26]. GALNT7 levels in tumour tissue are significantly reduced after neoadjuvant hormonal therapy (NHT) (n = 162, unpaired t test, p < 0.0001). Scale bar is 100 µm. B Analysis of a 125 case TMA reveals the levels of GALNT7 in hormone naïve and castrate resistant prostate cancer (CRPC) are similar. Scale bar is 100 µm. C GALNT7 serum levels significantly decrease following ADT (n = 20, Mann Whitney U test, p = 0.0039). D The serum levels of GALNT7 in men with mCRPC are significantly higher than in men with hormone naïve prostate cancer (n = 260, unpaired t test, p < 0.001). E In the SU2C mCRPC patient cohort (n = 163) [33, 80] GALNT7 mRNA levels correlate with the androgen response pathway (NES 2.45; FDR q value = <0.001).
GALNT7 can modify O-glycosylation in prostate cancer cells
GALNT7 was recently identified as a single gene effector of cell surface glycosylation [23]. However, how GALNT7 impacts glycosylation in prostate cancer cells has not been previously studied. GALNT enzymes are responsible for the initiation of GalNAc O-linked glycosylation and the synthesis of α-GalNAc1,3-O-Ser/Thr, or the Tn antigen. This structure is normally extended by the further sequential action of glycosyltransferases to build more complex O-linked structures, but in cancer the Tn antigen is often left unelaborated, and its presence is associated with poor patient prognosis [37]. Based on this, we hypothesised that upregulation of GALNT7 in prostate cancer cells would alter O-linked glycosylation, including exposure of truncated O-glycans such as the cancer-associated Tn antigen. To test if upregulation of GALNT7 can alter the cell surface O-glycosylation of prostate cancer cells, we created and validated stable prostate cancer cell line models with knockdown or up-regulation of GALNT7 (Supplementary Figure 5) and assessed the recognition by lectins. Lectin arrays and flow cytometry revealed that GALNT7 overexpression correlates with increased binding of SBA lectin (which recognises the cancer-associated Tn antigen [38, 39]) (Fig. 4A and Supplementary Fig. 6). This finding was confirmed via immunocytochemistry using an antibody specific to the Tn antigen (Fig. 4B), indicating that upregulation of GALNT7 introduces tumour-associated glycans on prostate cancer cells.
A Lectin flow cytometry shows PC3 cells with upregulated GALNT7 have increased binding to SBA lectin (which recognises terminal GalNAc or Tn antigen). B Detection of the cancer-associated Tn antigen by immunofluorescence in DU145 cells show upregulation of GALNT7 promotes increased expression of the Tn antigen. C Analysis of extracellular vesicles (EVs) in conditioned media from LNCaP and DU145 cells with knockdown or overexpression of GALNT7 suggests a correlation between GALNT7 and the Tn antigen in the prostate cancer secretome. D Detection of glycosylation of secreted proteins in samples from PC3 control cells or GALNT7-KO cells using GALNT7 bump-and-hole engineering. The active site of GALNT7 was engineered by mutation (IIe and Leu to 2xAla). The mutant (termed BH) uses a chemically modified analogue of the native substrate UDP-GalNAc. Following glycosylation, the chemical modification can be traced by methods of click chemistry.
As over 80% of the secretome is O-glycosylated [40], we predicted that upregulation of GALNT7 will also alter the secretome of prostate cancer cells. To investigate GALNT7-mediated O-glycosylation in the secretory pathway, we analysed conditioned media samples using lectin/antibody immunoassays, an implemented GALNT7 chemical biology reporter system [41], and O-glycoproteomics. Lectin/antibody profiling of extracellular vesicles (EVs), isolated from conditioned media, revealed increased levels of the Tn antigen when GALNT7 is upregulated, and reduced levels of Tn antigen when GALNT7 is downregulated (Fig. 5C and Supplementary Figure 7). Next, we established a GALNT7 chemical biology reporter system for the activity of the GALNT7 enzyme based on a tactic termed ‘bump-and-hole engineering’. Breifly, we engineered GALNT7 by mutation such that the resulting mutant (‘BH’ for bump-and-hole) recognises a chemically modified version of the activated sugar UDP-GalNAc [41, 42] (Fig. 4D). Following glycosylation by BH-GALNT7, the chemical modification can then be reacted with a biotin reporter probe using click chemistry [41,42,43,44]. Using the BH-GALNT7 reporter, we report specifically GALNT7 dependent glycosylation of secreted proteins in prostate cancer cells (Fig. 4D). Next, we analysed the O-glycoproteome in secretomes of DU145 prostate cancer cells with upregulated GALNT7 using mass spectrometry. This identified 34 glycopeptides as potential substrates for the GALNT7 enzyme in prostate cancer cells, the Tn antigen being the most abundant O-glycosylated form (Supplementary Figure 8). Taken together, our findings reveal that in prostate cancer cells, GALNT7 can modify the O-glycosylation of both cell surface proteins and proteins in the secretory pathway, leading to upregulation of the Tn antigen, which is a hallmark of cancer and involved in tumor progression and metastasis [38].
A Knockdown of GALNT7 using shRNA significantly reduces the growth of CWR22RV1 tumours xenografts in a subcutaneous xenograft model. B Upregulation of GALNT7 in PC3 cells significantly increases the growth of subcutaneous xenograft tumours. C Knockdown of GALNT7 decreases prostate cancer cell invasion and upregulation of GALNT7 promotes prostate cancer cell invasion. D RNAseq analysis of CWR22RV1 cells with knockdown of GALNT7 and DU145 cells with upregulated GALNT7 identified 457 genes that dynamically change in response to GALNT7. Gene Ontology analysis of the 457 genes regulated by GALNT7 shows an enrichment of genes with roles in the ‘cell cycle’ and a de-enrichment of genes with roles in ‘immune signalling’. E Proteomics analysis of DU145 cells with upregulation of GALNT7 identified 249 proteins with a significant change in expression levels. Revigo analysis of these 249 proteins identified ‘cell cycle’ and ‘immune signalling’ as significantly enriched pathways. F Analysis of the SU2C mCRPC cohort [33] identified immune related pathways (including interferon gamma response, interferon alpha response, complement, allograft rejection, IL6 signalling, TNFA Signaling via NFKB and inflammatory response) with attenuated enrichment with GALNT7 expression.
GALNT7 promotes prostate tumour growth and correlates with cell cycle and immune signalling pathways
We next investigated the effects of GALNT7 on the biology of prostate cancer cells showing that knockdown of GALNT7 inhibits prostate cancer cell proliferation and colony formation in vitro, whereas overexpression of GALNT7 has the opposite effect (Supplementary Fig. 9A–D). Using sub-cutaneous in vivo mouse models, we found that knockdown of GALNT7 significantly suppressed the growth of CWR22RV1 tumours (Fig. 5A), whereas overexpression of GALNT7 significantly increased the growth of PC3 tumours (Fig. 5B). Furthermore, using in vitro assays we show GALNT7 promotes prostate cancer cell migration and invasion (Fig. 5C and Supplementary Fig. 9E–G). Next, we analysed the effects of conditioned media on prostate cancer cell biology. These experiments showed that conditioned media from prostate cancer cells with upregulated GALNT7 significantly enhanced cell proliferation and colony formation of wildtype prostate cancer cells, indicating that this effect was likely mediated by the secreted GALNT7, other unknown proteins of the media or others molecules (Supplementary Fig. 9H, I). Taken together, the above data suggest that upregulation of GALNT7 promotes aggressive prostate cancer cell behaviour and tumour growth.
To identify signalling pathways controlled by GALNT7 in prostate cancer cells, we used RNA-sequencing to search for genes that change with either knockdown or upregulation of GALNT7. Bioinformatic analyses identified 457 genes that respond to GALNT7 levels (Supplementary Tables 2–4 and Supplementary Fig. 10A, B) with enrichment in the cell cycle processes and depletion in immune signalling pathways (Fig. 5D and Supplementary Fig. 10C). By analysing the proteome of GALNT7 overexpressing cells using mass spectrometry we identified 249 differentially expressed proteins, and also highlighted ‘cell cycle’ and ‘immune signalling’ as significantly altered by GALNT7 (Fig. 5E and Supplementary Table 5). Of particular interest, expression levels of the tumour suppressor gene FOXO1 were increased upon knockdown of GALNT7, and decreased when GALNT7 is overexpressed. FOXO1 is an important negative regulator of the cell cycle [45] that is often lost or downregulated in prostate cancer [46]. Validation at the protein level confirmed that upregulation of GALNT7 in prostate cancer cells promotes loss of FOXO1 protein (Supplementary Fig. 10D). Analysis of The Cancer Genome Atlas Prostate Adenocarcinoma (TCGA PRAD) cohort [24] revealed a significant correlation between the GALNT7 and FOXO1 genes in clinical prostate cancer tissue (Supplementary Fig. 10E). Furthermore, analysis of the metastatic CRPC Stand Up to Cancer (SU2C) cohort [33] identified seven ‘immune signalling’ pathways which negatively correlate with GALNT7 gene expression levels in clinical samples (Fig. 5F and Supplementary Table 1).
Discussion
Prostate cancer is the most common cancer in men and is a major clinical burden. New diagnostic tests and therapeutic options are urgently needed and could aid in patient stratification and improve patient quality of life and survival times. In prostate cancer, aberrant glycosylation is closely linked to a malignant phenotype, however the mechanisms driving these changes are unclear. An increased understanding of how altered glycosylation contributes to prostate cancer pathology will uncover an untapped resource of biomarkers and therapeutic targets, and has the potential to transform how the disease is diagnosed and treated.
In this study, we measured levels of the glycosylation enzyme GALNT7 in prostate cancer tissue, urine and blood samples and identified GALNT7 as being significantly upregulated in prostate cancer. By analysing tumour and blood samples from men with prostate cancer, we further show that GALNT7 levels remain high in castrate resistant prostate cancer, thus suggesting GALNT7 is increased during both the development of prostate cancer and the progression to relapsed treatment resistant disease. Traditionally, the diagnosis of prostate cancer has relied on the detection of prostate specific antigen (PSA) in patient blood, and tissue biopsy tests - however this is now widely accepted as being sub-optimal [8, 47]. The introduction of pre-biopsy mpMRI (multiparametric magnetic resonance imaging) has improved risk stratification for men with suspected prostate cancer [8, 48, 49], but there remains space for novel serum and urine biomarkers in this new pathway. In particular, many patients with Likert 3 disease on mpMRI still undergo biopsy but do not have clinically significant prostate cancer [32]. Here, we demonstrate urine GALNT7 can identify men with prostate cancer with improved accuracy than serum PSA levels, and show that combining GALNT7 with PSA further improves diagnostic performance. Furthermore, in mpMRI Likert 3 patients, we show urine GALNT7 holds promise to identify patients with clinically significant disease. Based on these data, we propose that GALNT7 is upregulated in prostate cancer and could be developed further as a as a non-invasive diagnostic biomarker.
Our findings also show GALNT7 can modify the O-glycosylation of both cell surface proteins and proteins in the secretory pathway, and identify GALNT7 as a key driver of tumour growth which correlates with cell cycle and immune signalling pathways in prostate cancer cells. These data are consistent with the recent finding that GALNT7 is a single gene effector of cell surface glycosylation and glycocalyx height [23], and suggest GALNT7 mediated O-glycosylation may act at the interface of important signalling pathways in prostate cancer cells to promote disease progression. Based on these findings, we propose GALNT7 is a key player in prostate cancer that can likely be exploited for therapeutic usage. Targeting aberrant glycosylation holds huge potential for cancer research [50,51,52], and we envisage that both GALNT7, and its associated glycans (particularly the cancer-associated Tn antigen) hold huge promise in prostate cancer therapy that needs to be explored.
Strategies to target glycosylation in cancer include, the use of carbohydrate analogues, the development of glycan specific chimeric antigen receptor (CAR) T, and the discovery of small molecule inhibitors with potential clinical applications [50]. Glycosyltransferase enzymes such as GALNT7 have the potential to be used for the discovery of effective cancer drugs. Specific inhibitors of GALNT enzymes are lacking, but an isozyme-selective inhibitor targeting GALNT3 was recently developed and shown to block the invasion of breast cancer cells with no toxic effects [53], thus paving the way for the discovery of small molecule modulators that control the activity of specific GALNTs. Moving forward, we anticipate the development of both isozyme and pan-specific modulators targeting GALNT7 will yield new inhibitors, conceptually related to the widespread use of drugs targeting protein kinases, to provide a new class of therapeutics to treat prostate cancer.
The data presented indicates upregulation of GALNT7 may increase the expression of the Tn antigen in prostate cancer. Tn is an important tumour-associated glycan and an established druggable target for several cancer types [15, 32, 33]. Studies show the Tn antigen is limited to cancers and is not expressed in healthy/normal tissue [54, 55]. Potential strategies to target Tn for cancer therapy include monoclonal antibodies [56], therapeutic vaccination [57,58,59], and engineered CAR T cells against Tn antigen on MUC1 [60]. Emerging research shows the Tn antigen contributes to an immune suppressive microenvironment and is a promising target for immunotherapy [32, 61,62,63,64]. Consistent with this, our findings indicate GALNT7 correlates with immune signalling pathways in prostate cancer. As Tn expression has been detected in up to 90% of prostate tumours [58, 65,66,67] there is huge potential to exploit this biology to benefit patients.
The absence of a glycosylation consensus sequence and the variability of glycan elaboration have historically made O-GalNAc glycans challenging to study. Hence, little is known about the protein substrate specificity of GALNT7, other than its requirement for previously O-glycosylated substrates [21, 68]. Here, we equipped cells with the ability to tag protein substrates of GALNT7 and report GALNT7 dependent glycosylation of secreted proteins in living prostate cancer cells. Alongside this, we performed site specific O-glycoproteomics to identify 34 potential protein substrates for GALNT7. It is tempting to speculate that the enhancement of prostate cancer cell proliferation by GALNT7 is likely due to either the activity of secreted GALNT7, or some of its protein substrates’ roles or combined functions. Taken together, our findings suggest that further characterisation of GALNT7 mediated O-glycosylation, alongside a systematic study of O-glycosylation in prostate tumours, will reveal novel biomarkers and potential therapeutic targets to provide clinically actionable information that could impact men with prostate cancer.
Methods
Cell culture and creation of stable cell lines
Cell culture and the cell lines used were as described previously [69]. The stable cell lines used in the study were created by lentiviral transduction using an MOI of 5. For details of the lentiviral particles used please see supplementary table 6. To generate GALNT7 KO cells guide RNAs ACATGAGGCCATGGTACCAC and GTACCATGGCCTCATGTTGA were cloned into the LentiCRISPRv2 plasmid (Addgene plasmid 52961). Lentivirus production, transduction and selection of monoclonal cell lines was carried out as previously described [70]. Cell lines were obtained from ATCC, authenticated using DNA STR analysis, and tested every 3 months for mycoplasma contamination.
Western blotting
Western blotting was performed as previously described [69]. For details of the antibodies used please see Supplementary Table 6.
Quantitative PCR
Quantitative PCR (qPCR) was performed as previously described [69]. For details of the primers used please see Supplementary Table 6.
Immunohistochemistry
For details of the TMAs tested and methods used please see Supplementary Table 7.
Detection of GALNT7 in urine and serum
Human GALNT7 sandwich pre-validated ELISA kits were purchased from Cambridge Bioscience (RayBioTech, ELH-GALNT7-1). Samples and standards were assayed in duplicate according to the manufacturer’s protocol. For specific details of the methods used for each cohort please see see Supplementary Table 8.
In vitro cell behavior assays
Cell proliferation assays were carried out using the WST-1 96 well Cell Proliferation Assay Kit (Cambridge Bioscience, CAY10008883) as per the manufacturer’s instructions. Colony formation assays were performed as previously described [19]. Invasion assays were carried out using the Oris Pro 96-well Invasion Assay (Amsbio, PROIA1) as per the manufacturer’s instructions.
Mouse models
CWR22RV1 tumour xenografts
Male NMRI mice (Charles Rivers) were inoculated at 8 weeks of age with 1 × 107 CWR22RV1 cells with GALNT7 knockdown by unilateral subcutaneous injection into the flank. The mice were randomised into control or treatment groups before cancer cell inoculation. Cells were injected in a volume of 100 µL cell culture media and Matrigel in a 1:1 mixture. Animals were weighed and tumour volumes monitored by caliper measurement three times a week by a blinded researcher until the first animal met a humane endpoint (defined as tumor volume reaching 2000 mm3). Tumors with ulceration were excluded from the analysis.
PC3 tumour xenografts
Male CD-1 Nude mice (Charles Rivers) were inoculated at 8 weeks of age with 1 × 107 PC3 cells with GALNT7 overexpression. The mice were randomised into control or treatment groups before cancer cell inoculation. Cells were injected in a volume of 50 µL of cell culture media and Matrigel in a 1:1 mixture. Animals were weighed and tumour volumes monitored by caliper measurement three times a week by an unblinded researcher until the first animal met a humane endpoint (defined as tumor volume reaching 1000 mm3). Tumors with ulceration were excluded from the analysis. Sample size estimate was based on our recent experience of similar xenograft studies. All animal work was conducted with project license approval granted by the UK Home Office and under the approval of the local biomedical research ethics committee.
Lectin array and lectin flow cytometry
The glycosylation profile of PC3 cells with upregulation of GALNT7 was monitored using a 26 lectin microarray kit (Z Biotech, 10606-K) according to the manufacturer’s instructions. Lectin flow cytometry was performed as described previously, using biotinylated SBA lectin (Vector labs, B-1015-5).
Isolation and analysis of Extracellular Vesicles
Detection of glycosylation changes of extracellular vesicles (EVs) from cell culture media was performed using a previously published nanoparticle based time-resolved fluorescence immunoassay (NP-TRFIA) [71] (further details are provided in Supplementary Fig. 8). The effect of GALNT7 KD or OE on glycosylations on EVs was calculated based on the signal-to-background (S/B) levels of Tn-assays. The CD63-CD63 signals were used to quantify the EV amount in each cell line.
GALNT7 bump-and-hole engineering and bioorthogonal labelling of secretome proteins by bump-and-hole engineered GALNT7
Bump- and-hole engineering of GALNT7 in living cells was performed based on previously established protocols [41]. For further details of the methods used see Supplementary Table 9.
Mass spectrometry-based proteomics and glycoproteomics
Mass spectrometry proteomics and glycoproteomics methods were adapted from previously described protocols [72,73,74]; for more details see Supplementary Table 10.
RNAseq and GO analysis
The RNA sequencing (RNA-seq) of cell lines was performed as described previously [19] and can be accessed on GEO repository (GSE220942). Data were further analysed using the gene ontology resource (http://geneontology.org) (accessed on 3 June 2020) [75, 76]. For details of how the SU2C/PCF mCRPC tissue cohort was analysed please see Supplementary Table 11.
Statistical analyses
All statistical analyses were performed using GraphPad Prisms 8 (GraphPad Software, Inc., San Diego, CA, USA). Data are presented as the mean of three independent samples ± standard deviation of the mean (SD). Unless otherwise stated, the variance between groups being statistically compared was similar. Statistical significance is indicated as *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
Data availability
The datasets generated during and analysed during this study are available in the figshare data repository. https://doi.org/10.6084/m9.figshare.21185902.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30.
Livermore K, Munkley J. DJ E. Androgen receptor and prostate cancer. AIMS Mol Sci. 2016;3:280–99.
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005.
Beer TM, Tombal B. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371:1755–6.
Morote J, Aguilar A, Planas J, Trilla E. Definition of Castrate Resistant Prostate Cancer: New Insights. Biomedicines. 2022;10:689.
Pinsky PF, Prorok PC, Kramer BS. Prostate Cancer Screening - A Perspective on the Current State of the Evidence. N Engl J Med. 2017;376:1285–9.
Attard G, Parker C, Eeles RA, Schroder F, Tomlins SA, Tannock I, et al. Prostate cancer. Lancet. 2016;387:70–82.
Ahmed HU, El-Shater Bosaily A, Brown LC, Gabe R, Kaplan R, Parmar MK, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815–22.
Scott E, Munkley J. Glycans as Biomarkers in Prostate Cancer. Int J Mol Sci. 2019;20:1389.
Varki A, Kornfeld S Historical Background and Overview. In: th, Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, et al., editors. Essentials of Glycobiology. Cold Spring Harbor (NY) 2022. p. 1–20.
Varki A, Gagneux P Biological Functions of Glycans. In: rd, Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, et al., editors. Essentials of Glycobiology. Cold Spring Harbor (NY) 2015. p. 77–88.
Chin-Hun Kuo J, Gandhi JG, Zia RN, Paszek MJ. Physical biology of the cancer cell glycocalyx. Nat Phys. 2018;14:658–69.
Paszek MJ, DuFort CC, Rossier O, Bainer R, Mouw JK, Godula K, et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature. 2014;511:319–25.
Buffone A, Weaver VM. Don’t sugarcoat it: How glycocalyx composition influences cancer progression. J Cell Biol. 2020;219:e201910070.
Munkley J, Mills IG, Elliott DJ. The role of glycans in the development and progression of prostate cancer. Nat Rev Urol. 2016;13:324–33.
Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–55.
Munkley J. Glycosylation is a global target for androgen control in prostate cancer cells. Endocr Relat Cancer. 2017;24:R49–R64.
Munkley J, Vodak D, Livermore KE, James K, Wilson BT, Knight B, et al. Glycosylation is an Androgen-Regulated Process Essential for Prostate Cancer Cell Viability. EBioMedicine. 2016;8:103–16.
Scott E, Garnham R, Cheung K, Duxfield A, Elliott DJ, Munkley J. Pro-Survival Factor EDEM3 Confers Therapy Resistance in Prostate Cancer. Int J Mol Sci. 2022;23:8184.
Munkley J, Oltean S, Vodak D, Wilson BT, Livermore KE, Zhou Y, et al. The androgen receptor controls expression of the cancer-associated sTn antigen and cell adhesion through induction of ST6GalNAc1 in prostate cancer. Oncotarget. 2015;6:34358–74.
Bennett EP, Mandel U, Clausen H, Gerken TA, Fritz TA, Tabak LA. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology. 2012;22:736–56.
de Las Rivas M, Lira-Navarrete E, Gerken TA, Hurtado-Guerrero R. Polypeptide GalNAc-Ts: from redundancy to specificity. Curr Opin Struct Biol. 2019;56:87–96.
Mockl L, Pedram K, Roy AR, Krishnan V, Gustavsson AK, Dorigo O, et al. Quantitative Super-Resolution Microscopy of the Mammalian Glycocalyx. Dev Cell. 2019;50:57–72.e6.
Hoadley KA, Yau C, Hinoue T, Wolf DM, Lazar AJ, Drill E, et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell. 2018;173:291–304.e6.
Burska UL, Harle VJ, Coffey K, Darby S, Ramsey H, O’Neill D, et al. Deubiquitinating enzyme Usp12 is a novel co-activator of the androgen receptor. J Biol Chem. 2013;288:32641–50.
Nouri M, Massah S, Caradec J, Lubik AA, Li N, Truong S, et al. Transient Sox9 Expression Facilitates Resistance to Androgen-Targeted Therapy in Prostate Cancer. Clin Cancer Res. 2020;26:1678–89.
Sun X, Mahajan D, Chen B, Song Z, Lu L. A quantitative study of the Golgi retention of glycosyltransferases. J Cell Sci. 2021;134:jcs258564.
Lichtenthaler SF, Lemberg MK, Fluhrer R. Proteolytic ectodomain shedding of membrane proteins in mammals-hardware, concepts, and recent developments. EMBO J. 2018;37:e99456.
Hirata T, Takata M, Tokoro Y, Nakano M, Kizuka Y. Shedding of N-acetylglucosaminyltransferase-V is regulated by maturity of cellular N-glycan. Commun Biol. 2022;5:743.
Hait NC, Maiti A, Wu R, Andersen VL, Hsu CC, Wu Y, et al. Extracellular sialyltransferase st6gal1 in breast tumor cell growth and invasiveness. Cancer Gene Ther. 2022;29:1662–75.
Johnston E, Pye H, Bonet-Carne E, Panagiotaki E, Patel D, Galazi M, et al. INNOVATE: A prospective cohort study combining serum and urinary biomarkers with novel diffusion-weighted magnetic resonance imaging for the prediction and characterization of prostate cancer. BMC Cancer. 2016;16:816.
Pye H, Singh S, Norris JM, Carmona Echeverria LM, Stavrinides V, Grey A, et al. Evaluation of PSA and PSA Density in a Multiparametric Magnetic Resonance Imaging-Directed Diagnostic Pathway for Suspected Prostate Cancer: The INNOVATE Trial. Cancers. 2021;13:1985.
Abida W, Cyrta J, Heller G, Prandi D, Armenia J, Coleman I, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci. 2019;116:11428–36.
Chen X, Miao Z, Divate M, Zhao Z, Cheung E. KM-express: an integrated online patient survival and gene expression analysis tool for the identification and functional characterization of prognostic markers in breast and prostate cancers. Database. 2018;2018:bay069.
Rajan P, Sudbery IM, Villasevil ME, Mui E, Fleming J, Davis M, et al. Next-generation sequencing of advanced prostate cancer treated with androgen-deprivation therapy. Eur Urol. 2014;66:32–9.
Akamatsu S, Wyatt AW, Lin D, Lysakowski S, Zhang F, Kim S, et al. The Placental Gene PEG10 Promotes Progression of Neuroendocrine Prostate Cancer. Cell Rep. 2015;12:922–36.
Beaman EM, Carter DRF, Brooks SA. GALNTs: master regulators of metastasis-associated epithelial-mesenchymal transition (EMT)? Glycobiology. 2022;32:556–79.
Radhakrishnan P, Dabelsteen S, Madsen FB, Francavilla C, Kopp KL, Steentoft C, et al. Immature truncated O-glycophenotype of cancer directly induces oncogenic features. Proc Natl Acad Sci. 2014;111:E4066–75.
Ju T, Aryal RP, Kudelka MR, Wang Y, Cummings RD. The Cosmc connection to the Tn antigen in cancer. Cancer Biomark. 2014;14:63–81.
Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013;32:1478–88.
Schumann B, Malaker SA, Wisnovsky SP, Debets MF, Agbay AJ, Fernandez D, et al. Bump-and-Hole Engineering Identifies Specific Substrates of Glycosyltransferases in Living Cells. Mol Cell. 2020;78:824–34.e15.
Cioce A, Malaker SA, Schumann B. Generating orthogonal glycosyltransferase and nucleotide sugar pairs as next-generation glycobiology tools. Curr Opin Chem Biol. 2021;60:66–78.
Choi J, Wagner LJS, Timmermans S, Malaker SA, Schumann B, Gray MA, et al. Engineering Orthogonal Polypeptide GalNAc-Transferase and UDP-Sugar Pairs. J Am Chem Soc. 2019;141:13442–53.
Debets MF, Tastan OY, Wisnovsky SP, Malaker SA, Angelis N, Moeckl LKR, et al. Metabolic precision labeling enables selective probing of O-linked N-acetylgalactosamine glycosylation. Proc Natl Acad Sci. 2020;117:25293–301.
Yuan C, Wang L, Zhou L, Fu Z. The function of FOXO1 in the late phases of the cell cycle is suppressed by PLK1-mediated phosphorylation. Cell Cycle. 2014;13:807–19.
Yang Y, Blee AM, Wang D, An J, Pan Y, Yan Y, et al. Loss of FOXO1 Cooperates with TMPRSS2-ERG Overexpression to Promote Prostate Tumorigenesis and Cell Invasion. Cancer Res. 2017;77:6524–37.
Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375:1415–24.
Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, et al. Systematic review of complications of prostate biopsy. Eur Urol. 2013;64:876–92.
Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-Targeted or Standard Biopsy for Prostate-Cancer Diagnosis. N Engl J Med. 2018;378:1767–77.
Costa AF, Campos D, Reis CA, Gomes C. Targeting Glycosylation: A New Road for Cancer Drug Discovery. Trends. Cancer 2020;6:757–66.
Mereiter S, Balmana M, Campos D, Gomes J, Reis CA. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell. 2019;36:6–16.
Munkley J, Scott E. Targeting Aberrant Sialylation to Treat Cancer. Medicines. 2019;6:102.
Song L, Linstedt AD. Inhibitor of ppGalNAc-T3-mediated O-glycosylation blocks cancer cell invasiveness and lowers FGF23 levels. Elife. 2017;6:e24051.
Romer TB, Aasted MKM, Dabelsteen S, Groen A, Schnabel J, Tan E, et al. Mapping of truncated O-glycans in cancers of epithelial and non-epithelial origin. Br J Cancer. 2021;125:1239–50.
Persson N, Stuhr-Hansen N, Risinger C, Mereiter S, Polonia A, Polom K, et al. Epitope mapping of a new anti-Tn antibody detecting gastric cancer cells. Glycobiology. 2017;27:635–45.
Morita N, Yajima Y, Asanuma H, Nakada H, Fujita-Yamaguchi Y. Inhibition of cancer cell growth by anti-Tn monoclonal antibody MLS128. Biosci Trends. 2009;3:32–7.
Beckwith DM, Cudic M. Tumor-associated O-glycans of MUC1: Carriers of the glyco-code and targets for cancer vaccine design. Semin Immunol. 2020;47:101389.
Li Q, Anver MR, Butcher DO, Gildersleeve JC. Resolving conflicting data on expression of the Tn antigen and implications for clinical trials with cancer vaccines. Mol Cancer Ther. 2009;8:971–9.
Slovin SF, Ragupathi G, Musselli C, Olkiewicz K, Verbel D, Kuduk SD, et al. Fully synthetic carbohydrate-based vaccines in biochemically relapsed prostate cancer: clinical trial results with alpha-N-acetylgalactosamine-O-serine/threonine conjugate vaccine. J Clin Oncol. 2003;21:4292–8.
Posey AD Jr, Schwab RD, Boesteanu AC, Steentoft C, Mandel U, Engels B, et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity. 2016;44:1444–54.
Freire T, Lo-Man R, Bay S, Leclerc C. Tn glycosylation of the MUC6 protein modulates its immunogenicity and promotes the induction of Th17-biased T cell responses. J Biol Chem. 2011;286:7797–811.
Scott E, Elliott DJ, Munkley J. Tumour associated glycans: A route to boost immunotherapy? Clin Chim Acta. 2020;502:167–73.
Cornelissen LAM, Blanas A, Zaal A, van der Horst JC, Kruijssen LJW, O’Toole T, et al. Tn Antigen Expression Contributes to an Immune Suppressive Microenvironment and Drives Tumor Growth in Colorectal Cancer. Front Oncol. 2020;10:1622.
Nativi C, Papi F, Roelens S. Tn antigen analogues: the synthetic way to “upgrade” an attracting tumour associated carbohydrate antigen (TACA). Chem Commun. 2019;55:7729–36.
Zhang S, Zhang HS, Reuter VE, Slovin SF, Scher HI, Livingston PO. Expression of potential target antigens for immunotherapy on primary and metastatic prostate cancers. Clin Cancer Res. 1998;4:295–302.
Charpin C, Pancino G, Osinaga E, Bonnier P, Lavaut MN, Allasia C, et al. Monoclonal antibody 83D4 immunoreactivity in human tissues: cellular distribution and microcytophotometric analysis of immunoprecipitates on tissue sections. Anticancer Res. 1992;12:209–23.
Huang J, Byrd JC, Siddiki B, Yuan M, Lau E, Kim YS. Monoclonal antibodies against partially deglycosylated colon cancer mucin that recognize Tn antigen. Dis Markers. 1992;10:81–94.
Kong Y, Joshi HJ, Schjoldager KT, Madsen TD, Gerken TA, Vester-Christensen MB, et al. Probing polypeptide GalNAc-transferase isoform substrate specificities by in vitro analysis. Glycobiology. 2015;25:55–65.
Munkley J, Li L, Krishnan SRG, Hysenaj G, Scott E, Dalgliesh C, et al. Androgen-regulated transcription of ESRP2 drives alternative splicing patterns in prostate cancer. Elife. 2019;8:e47678.
Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–4.
Islam MK, Syed P, Lehtinen L, Leivo J, Gidwani K, Wittfooth S, et al. A Nanoparticle-Based Approach for the Detection of Extracellular Vesicles. Sci Rep. 2019;9:10038.
Bermudez A, Pitteri SJ. Enrichment of Intact Glycopeptides Using Strong Anion Exchange and Electrostatic Repulsion Hydrophilic Interaction Chromatography. Methods Mol Biol. 2021;2271:107–20.
Gatto L, Lilley KS. MSnbase-an R/Bioconductor package for isobaric tagged mass spectrometry data visualization, processing and quantitation. Bioinformatics. 2012;28:288–9.
Navarro P, Trevisan-Herraz M, Bonzon-Kulichenko E, Nunez E, Martinez-Acedo P, Perez-Hernandez D, et al. General statistical framework for quantitative proteomics by stable isotope labeling. J Proteome Res. 2014;13:1234–47.
Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–9.
Gene Ontology C. The Gene Ontology resource: enriching a GOld mine. Nucl Acids Res. 2021;49:D325–D34.
Kirkegaard T, Edwards J, Tovey S, McGlynn LM, Krishna SN, Mukherjee R, et al. Observer variation in immunohistochemical analysis of protein expression, time for a change? Histopathology. 2006;48:787–94.
Munkley J, McClurg UL, Livermore KE, Ehrmann I, Knight B, McCullagh P, et al. The cancer-associated cell migration protein TSPAN1 is under control of androgens and its upregulation increases prostate cancer cell migration. Sci Rep. 2017;7:5249.
Vidal I, Zheng Q, Hicks JL, Chen J, Platz EA, Trock BJ, et al. GSTP1 positive prostatic adenocarcinomas are more common in Black than White men in the United States. PLoS One. 2021;16:e0241934.
Sharp A, Coleman I, Yuan W, Sprenger C, Dolling D, Rodrigues DN, et al. Androgen receptor splice variant-7 expression emerges with castration resistance in prostate cancer. J Clin Invest. 2019;129:192–208.
Acknowledgements
This work was funded by Prostate Cancer UK through a Research Innovation Award and the Bob Willis Fund [RIA16-ST2-011], a Prostate Cancer UK Travelling Prize Fellowship [TLD-PF19-002], the JGW Patterson Foundation, and Prostate Cancer Research and The Mark Foundation for Cancer Research (grant reference 6961). The work performed at the Vancouver Prostate Centre was funded by the Terry Fox Research Institute (TFRI-NF-PPG). The research was supported / funded by the NIHR Exeter Clinical Research Facility. The opinions given in this paper do not necessarily represent those of the NIHR, the NHS or the Department of Health. This work is also supported by the Department of Defense Prostate Cancer Research Program, DOD Award No W81XWH-18-2-0013, W81XWH-18-2-0015, W81XWH-18-2-0016, W81XWH-18-2-0017, W81XWH-18-2-0018 and W81XWH-18-2-0019 PCRP Prostate Cancer Biorepository Network (PCBN). This work was supported by the NIH (R01 CA200423 and U01 CA196387), the Howard Hughes Medical Institute, Stanford Sarafan Chem-H and the Francis Crick Institute which receives its core funding from Cancer Research UK (FC001749), the UK Medical Research Council (FC001749) and Wellcome Trust (FC001749). BC was supported by a Crick-HEI studentship funded by the Department of Chemistry at Imperial College London and the Francis Crick Institute. PW was supported by a Banting Postdoctoral Fellowship from the Canadian Institutes of Health Research. MAG was supported by the National Science Foundation Graduate Research Fellowship and the Stanford ChEM-H Chemistry/Biology Interface Predoctoral Training Program. SW is supported by funding from the Canadian Institutes of Health Research, the Natural Sciences and Engineering Research Council of Canada, the Cancer Research Society and the Canadian Glycomics Network (GlycoNet). The expression plasmid of GALNT7 in pGen2-DEST (DNASU) was a gift from Kelley Moremen and generated through NIH grant RR005351. The authors would like to thank urology surgeon Mr Matthew Simms and tissue procurement officer Dr Vincent Mann for help collecting clinical samples, and Professor Fiona Oakley for assistance with our in vivo studies. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Author information
Authors and Affiliations
Contributions
ES, KH, BC, AB, FJGM, ECY, KI, MOM, KB, CR, MAG, LK, AT, EM, GH, EAG, RG and AD performed in vitro experiments. KC, DB, DW, WY and AS performed bio-informatic analyses. ES, HT, HMA and NW performed the in vivo studies. HP, FMF, SH, USF, AH, AF, SS, EWJ, SH, BK, PM, JM, MC, LH, PF, AS, JdB and HW contributed to clinical sample collection. ULM, LW and HZO performed IHC on tissue sections. HT and ULM scored pathology sections. MJM, AS, JdB, SW, CRB, MD, JL, HW, SP, DJE, NW and BS supervised the study and and/or provided critical review. JM wrote the manuscript. BS, ES, HW, RH, RHG, DJE and NW contributed to critical review and paper writing. ES and JM designed, analysed and interpreted the study. JM conceived the study and is senior author and corresponding author. All authors read the manuscript, agree with the content, and were given the opportunity to provide input.
Corresponding author
Ethics declarations
Competing interests
JM is shareholder and Scientific Advisor of GlycoScoreDx Ltd. ES and GH are shareholders of GlycoScoreDx Ltd. The authors have filed a patent relating to this work (GB Patent GB2,594,103). CRB is a cofounder and scientific advisory board member of Lycia Therapeutics, Palleon Pharmaceuticals, EnableBioscience, Redwood Biosciences (a subsidiary of Catalent), OliLux Bio, Grace Science LLC, and InterVenn Biosciences. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scott, E., Hodgson, K., Calle, B. et al. Upregulation of GALNT7 in prostate cancer modifies O-glycosylation and promotes tumour growth. Oncogene 42, 926–937 (2023). https://doi.org/10.1038/s41388-023-02604-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-023-02604-x
This article is cited by
-
Decoding dynamic miRNA:ceRNA interactions unveils therapeutic insights and targets across predominant cancer landscapes
BioData Mining (2024)
-
Metabolic adaptations in prostate cancer
British Journal of Cancer (2024)
-
Glycosylation: mechanisms, biological functions and clinical implications
Signal Transduction and Targeted Therapy (2024)
-
ST3 beta-galactoside alpha-2,3-sialyltransferase 1 (ST3Gal1) synthesis of Siglec ligands mediates anti-tumour immunity in prostate cancer
Communications Biology (2024)
-
The role of GALNT7 as a potential diagnostic marker in prostate cancer
Nature Reviews Urology (2023)