Abstract
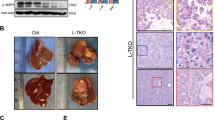
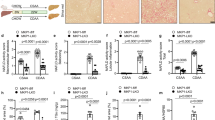
Hepatocellular carcinoma (HCC) is the most common form of primary liver cancer. Accumulating evidence indicates that non-alcoholic steatohepatitis (NASH) is a key predisposing factor for HCC occurrence. However, the precise mechanisms driving NASH transition to HCC remain largely obscure. Augmenter of liver regeneration (ALR) is a sulfhydryl oxidase and cytochrome c reductase that functions as an important regulator of mitochondrial dynamics. In this study, we focused on ALR ubiquitination-mediated degradation and its potential contribution to NASH-driven HCC progression at the mitochondrial level. Hepatic ALR expression in HCC patients was determined using immunohistochemical analysis. Mice with liver-specific deletion of ALR (ALRCKO) and ALRWT mice were fed a western diet (WD) and high-sugar solution for induction of NASH. HCC in animals was induced via peritoneal administration of CCl4. ALR expression was markedly decreased in liver tissues of patients with NASH and HCC compared with non-NASH and non-tumor tissues. Similarly, in ALRWT mice, the ALR level in tumor tissue was reduced relative to that in para-tumor tissue. In the ALRCKO group, mice fed WD plus CCl4 developed HCC starting at week 12 while ALRWT mice fed WD plus CCl4 developed HCC at week 24. Analysis of protein posttranslational modifications revealed ubiquitylation (Ub) and deubiquitination (DUb) of ALR by murine double minute 2 (MDM2) and ubiquitin-specific protease 36 (USP36), respectively. Imbalance between Ub and DUb of ALR resulted in profound ALR degradation, which appeared to be reversibly associated with Edmondson-Steiner tumor grade. Rescue of ALR levels via gene transfection abolished tumor malignant features to a certain extent in vitro. Notably, ALR deletion substantially enhanced mitochondrial fission by activating Drp1 phosphorylation at Ser616, thus disrupting the balance of mitochondrial dynamics between fission and fusion and severely impairing oxidative phosphorylation (OXPHOS) and ATP synthesis, instead enhancing anaerobic metabolism, which might be attributed to steatotic hepatocyte transition into the malignant HCC phenotype. Hepatic ALR depletion via dysregulation of ubiquitination is a critical aggravator of NASH-HCC progression and represents a promising therapeutic target for related liver diseases.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The RNA-seq raw data of this study are available from the Sequence Read Archive repository BioProject (https://www.ncbi.nlm.nih.gov/bioproject/) with accession number PRJNA841174. Immunopurification and Mass spectrometry raw data have been deposited to the PRIDE database (https://www.iprox.cn/page/SSV024.html;url=1651495320815MEBa, the accession number is IPX0004307003. Please access the raw files with password “bzme”). Other materials, data, and any associated protocols that support the findings of this study are available from the corresponding authors on reasonable request.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Younossi ZM, Otgonsuren M, Henry L, Venkatesan C, Mishra A, Erario M, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62:1723–30.
Hashimoto E, Tokushige K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: Growing evidence of an epidemic? Hepatol Res. 2012;42:1–14.
Sung H, Siegel RL, Torre LA, Pearson-Stuttard J, Islami F, Fedewa SA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin. 2019;69:88–112.
Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384–91.
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–57.
Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208.
Tilg H, Hotamisligil GS. Nonalcoholic fatty liver disease: Cytokine-adipokine interplay and regulation of insulin resistance. Gastroenterology. 2006;131:934–45.
Nakagawa H, Umemura A, Taniguchi K, Font-Burgada J, Dhar D, Ogata H, et al. ER stress cooperates with hypernutrition to trigger TNF-dependent spontaneous HCC development. Cancer Cell. 2014;26:331–43.
Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101.
Nakagawa H. Recent advances in mouse models of obesity- and nonalcoholic steatohepatitis-associated hepatocarcinogenesis. World J Hepatol. 2015;7:2110–18.
LaBrecque DR, Pesch LA. Preparation and partial characterization of hepatic regenerative stimulator substance (SS) from rat liver. J Physiol. 1975;248:273–84.
Mei MH, An W, Zhang BH, Shao Q, Gong DZ. Hepatic stimulator substance protects against acute liver failure induced by carbon tetrachloride poisoning in mice. Hepatology. 1993;17:638–44.
Ho DW, Fan ST, To J, Woo YH, Zhang Z, Lau C, et al. Selective plasma filtration for treatment of fulminant hepatic failure induced by D-galactosamine in a pig model. Gut. 2002;50:869–76.
Wu Y, Chen L, Yu H, Liu H, An W. Transfection of hepatic stimulator substance gene desensitizes hepatoma cells to H2O2-induced cell apoptosis via preservation of mitochondria. Arch Biochem Biophysics. 2007;464:48–56.
Yu HY, Zhu MH, Xiang DR, Li J, Sheng JF. High expression of 23 kDa protein of augmenter of liver regeneration (ALR) in human hepatocellular carcinoma. Onco Targets Ther. 2014;7:887–93.
Cao Y, Fu YL, Yu M, Yue PB, Ge CH, Xu WX, et al. Human augmenter of liver regeneration is important for hepatoma cell viability and resistance to radiation-induced oxidative stress. Free Radic Biol Med. 2009;47:1057–66.
Thasler WE, Schlott T, Thelen P, Hellerbrand C, Bataille F, Lichtenauer M, et al. Expression of augmenter of liver regeneration (ALR) in human liver cirrhosis and carcinoma. Histopathology. 2005;47:57–66.
Dayoub R, Wagner H, Bataille F, Stöltzing O, Spruss T, Buechler C, et al. Liver regeneration associated protein (ALR) exhibits antimetastatic potential in hepatocellular carcinoma. Mol Med (Camb, Mass). 2011;17:221–28.
Gandhi CR, Chaillet JR, Nalesnik MA, Kumar S, Dangi A, Demetris AJ, et al. Liver-specific deletion of augmenter of liver regeneration accelerates development of steatohepatitis and hepatocellular carcinoma in mice. Gastroenterology. 2015;148:379–91.
Hernández-Alvarez MI, Sebastián D, Vives S, Ivanova S, Bartoccioni P, Kakimoto P, et al. Deficient endoplasmic reticulum-mitochondrial phosphatidylserine transfer causes liver disease. Cell. 2019;177:881–95.
Fujiwara N, Nakagawa H, Enooku K, Kudo Y, Hayata Y, Nakatsuka T, et al. CPT2 downregulation adapts HCC to lipid-rich environment and promotes carcinogenesis via acylcarnitine accumulation in obesity. Gut. 2018;67:1493–504.
Tsuchida T, Lee YA, Fujiwara N, Ybanez M, Allen B, Martins S, et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J Hepatol. 2018;69:385–95.
Li Y, Xie P, Lu L, Wang J, Diao L, Liu Z, et al. An integrated bioinformatics platform for investigating the human E3 ubiquitin ligase-substrate interaction network. Nat Commun. 2017;8:347.
Wang X, Li Y, He M, Kong X, Jiang P, Liu X, et al. UbiBrowser 2.0: a comprehensive resource for proteome-wide known and predicted ubiquitin ligase/deubiquitinase-substrate interactions in eukaryotic species. Nucleic Acids Res. 2022;50:D719–28.
Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–44.
Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–33.
Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2021;18:223–38.
Jia X, Li Z, Dong L, Sun G, Wang X, Gao J, et al. Lack of hepatic stimulator substance expression promotes hepatocellular carcinoma metastasis partly through ERK-activated epithelial-mesenchymal transition. Lab Investig J Tech Methods Pathol. 2018;98:871–82.
Shi H, Han W, Shi H, Ren F, Chen D, Chen Y. et al. Augmenter of liver regeneration protects against carbon tetrachloride-induced liver injury by promoting autophagy in mice. Oncotarget. 2017;8:12637–48.
Wang N, Sun H, Tang L, Deng J, Luo Y, Guo H, et al. Establishment and primary clinical application of competitive inhibition for measurement of augmenter of liver regeneration. Exp Therapeutic Med. 2014;7:93–6.
Nalesnik MA, Gandhi CR, Starzl TE. Augmenter of liver regeneration: A fundamental life protein. Hepatology. 2017;66:266–70.
Lane DP, Hall PA. MDM2–arbiter of p53’s destruction. Trends Biochem Sci. 1997;22:372–74.
Kim MS, Ramakrishna S, Lim KH, Kim JH, Baek KH. Protein stability of mitochondrial superoxide dismutase SOD2 is regulated by USP36. J Cell Biochem. 2011;112:498–508.
Begriche K, Massart J, Robin MA, Bonnet F, Fromenty B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology. 2013;58:1497–507.
Galloway CA, Lee H, Brookes PS, Yoon Y. Decreasing mitochondrial fission alleviates hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2014;307:G632–41.
Du J, Zhang X, Han J, Man K, Zhang Y, Chu ES, et al. Pro-Inflammatory CXCR3 Impairs Mitochondrial Function in Experimental Non-Alcoholic Steatohepatitis. Theranostics. 2017;7:4192–203.
Archer SL. Mitochondrial dynamics–mitochondrial fission and fusion in human diseases. N. Engl J Med. 2013;369:2236–51.
Mansouri A, Gattolliat CH, Asselah T. Mitochondrial dysfunction and signaling in chronic liver diseases. Gastroenterology. 2018;155:629–47.
Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–25.
Zhang C, Huang J, An W. Hepatic stimulator substance resists hepatic ischemia/reperfusion injury by regulating Drp1 translocation and activation. Hepatology. 2017;66:1989–2001.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) (No. 32071128).
Author information
Authors and Affiliations
Contributions
Study concept and design: XP, AW; acquisition of patient specimens: ZMZ, GYY; acquisition of data: ZMZ, AZW, CYJ; analysis and interpretation of data: ZMZ, XP; drafting of the manuscript: ZMZ; study supervision and manuscript revision: AW; PI of project and lead contact: AW.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Capital Medical University Ethics Committee.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, M., Ai, Z., Guo, Y. et al. Imbalance in ALR ubiquitination accelerates the progression of nonalcoholic steatohepatitis to hepatocellular carcinoma. Oncogene 42, 308–321 (2023). https://doi.org/10.1038/s41388-022-02549-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02549-7