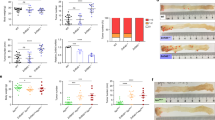
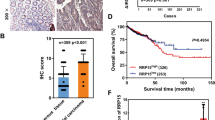
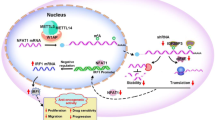
Abstract
Extensive protein synthesis is necessary for uncontrolled cancer cell proliferation, requiring hyperactive ribosome biogenesis. Our previous Pan-cancer study has identified EXOSC8 as a potential copy number variation (CNV)-driven rRNA metabolism-related oncogene in colorectal cancer (CRC). Herein, we further investigated proliferation-prompting functions and mechanisms of EXOSC8 in CRC by performing in silico analyses and wet-lab experiments. We uncovered that increased EXOSC8 expression and CNV levels are strongly associated with ribosome biogenesis-related factor levels in CRC, including ribosome proteins (RPs), eukaryotic translation initiation factors and RNA polymerase I/III. EXOSC8 silence decreases nucleolar protein and proliferation marker levels, as well as rRNA/DNA and global protein syntheses. Clinically, EXOSC8 is upregulated across human cancers, particularly CNV-driven upregulation in CRC was markedly associated with poor clinical outcomes. Mechanistically, EXOSC8 knockdown increased p53 levels in CRC, and the oncogenic proliferation phenotypes of EXOSC8 depended on p53 in vitro and in vivo. We discovered that EXOSC8 knockdown in CRC cells triggers ribosomal stress, nucleolar RPL5/11 being released into the nucleoplasm and “hijacking” Mdm2 to block its E3 ubiquitin ligase function, thus releasing and activating p53. Furthermore, our therapeutic experiments provided initial evidence that EXOSC8 might serve as a potential therapeutic target in CRC. Our findings revealed, for the first time, that the RNA exosome gene (EXOSC8) promotes CRC tumorigenesis by regulating cancer-related ribosome biogenesis in CRC. This study further extends our previous Pan-cancer study of the rRNA metabolism-related genes. The inhibition of EXOSC8 is a novel therapeutic strategy for the RPs-Mdm2-p53 ribosome biogenesis surveillance pathway in CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Kang J, Brajanovski N, Chan KT, Xuan J, Pearson RB, Sanij E. Ribosomal proteins and human diseases: molecular mechanisms and targeted therapy. Signal Transduct Target Ther. 2021;6:323.
Cui K, Liu C, Li X, Zhang Q, Li Y. Comprehensive characterization of the rRNA metabolism-related genes in human cancer. Oncogene 2020;39:786–800.
Boczonadi V, Muller JS, Pyle A, Munkley J, Dor T, Quartararo J, et al. EXOSC8 mutations alter mRNA metabolism and cause hypomyelination with spinal muscular atrophy and cerebellar hypoplasia. Nat Commun. 2014;5:4287.
Muller JS, Burns DT, Griffin H, Wells GR, Zendah RA, Munro B, et al. RNA exosome mutations in pontocerebellar hypoplasia alter ribosome biogenesis and p53 levels. Life Sci Alliance. 2020;3:e202000678.
Liu Q, Xiao Q, Sun Z, Wang B, Wang L, Wang N, et al. Exosome component 1 cleaves single-stranded DNA and sensitizes human kidney renal clear cell carcinoma cells to poly(ADP-ribose) polymerase inhibitor. Elife. 2021;10:e69454.
Taniue K, Tanu T, Shimoura Y, Mitsutomi S, Han H, Kakisaka R, et al. RNA Exosome Component EXOSC4 Amplified in Multiple Cancer Types Is Required for the Cancer Cell Survival. Int J Mol Sci. 2022;23:496.
Chen X, Huang Y, Liu J, Lin W, Chen C, Chen Y, et al. EXOSC5 promotes proliferation of gastric cancer through regulating AKT/STAT3 signaling pathways. J Cancer. 2022;13:1456–67.
Pan H, Pan J, Song S, Ji L, Lv H, Yang Z. EXOSC5 as a Novel Prognostic Marker Promotes Proliferation of Colorectal Cancer via Activating the ERK and AKT Pathways. Front Oncol. 2019;9:643.
Prakash V, Carson BB, Feenstra JM, Dass RA, Sekyrova P, Hoshino A, et al. Ribosome biogenesis during cell cycle arrest fuels EMT in development and disease. Nat Commun. 2019;10:2110.
Hong F, Meng Q, Zhang W, Zheng R, Li X, Cheng T, et al. Single-Cell Analysis of the Pan-Cancer Immune Microenvironment and scTIME Portal. Cancer Immunol Res. 2021;9:939–51.
Pelletier J, Thomas G, Volarevic S. Ribosome biogenesis in cancer: new players and therapeutic avenues. Nat Rev Cancer. 2018;18:51–63.
Morral C, Stanisavljevic J, Hernando-Momblona X, Mereu E, Alvarez-Varela A, Cortina C, et al. Zonation of Ribosomal DNA Transcription Defines a Stem Cell Hierarchy in Colorectal Cancer. Cell Stem Cell. 2020;26:845–861.e812.
Zanchin NI, Goldfarb DS. The exosome subunit Rrp43p is required for the efficient maturation of 5.8S, 18S and 25S rRNA. Nucl Acids Res. 1999;27:1283–8.
Garden GA, Hartlage-Rubsamen M, Rubel EW, Bothwell MA. Protein masking of a ribosomal RNA epitope is an early event in afferent deprivation-induced neuronal death. Mol Cell Neurosci. 1995;6:293–310.
Boutelle AM, Attardi LD. p53 and Tumor Suppression: It Takes a Network. Trends Cell Biol. 2021;31:298–310.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci. 2005;102:15545–50.
Deisenroth C, Zhang Y. Ribosome biogenesis surveillance: probing the ribosomal protein-Mdm2-p53 pathway. Oncogene 2010;29:4253–60.
Hein N, Hannan KM, George AJ, Sanij E, Hannan RD. The nucleolus: an emerging target for cancer therapy. Trends Mol Med. 2013;19:643–54.
Jhan YY, Prasca-Chamorro D, Palou Zuniga G, Moore DM, Arun Kumar S, Gaharwar AK, et al. Engineered extracellular vesicles with synthetic lipids via membrane fusion to establish efficient gene delivery. Int J Pharm. 2020;573:118802.
Ferdows BE, Patel DN, Chen W, Huang X, Kong N, Tao W. RNA cancer nanomedicine: nanotechnology-mediated RNA therapy. Nanoscale 2022;14:4448–55.
Zhang Y, Liu Q, Zhang X, Huang H, Tang S, Chai Y, et al. Recent advances in exosome-mediated nucleic acid delivery for cancer therapy. J Nanobiotechnology. 2022;20:279.
Yang B, Chen Y, Shi J. Exosome Biochemistry and Advanced Nanotechnology for Next-Generation Theranostic Platforms. Adv Mater. 2019;31:e1802896.
Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–5.
van Eijndhoven MAJ, Baglio SR, Pegtel DM. Packaging RNA drugs into extracellular vesicles. Nat Biomed Eng. 2020;4:6–8.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49.
Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, et al. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. 2022;2:1–9.
Morton DJ, Kuiper EG, Jones SK, Leung SW, Corbett AH, Fasken MB. The RNA exosome and RNA exosome-linked disease. RNA 2018;24:127–42.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646–74.
Shen A, Chen Y, Liu L, Huang Y, Chen H, Qi F, et al. EBF1-Mediated Upregulation of Ribosome Assembly Factor PNO1 Contributes to Cancer Progression by Negatively Regulating the p53 Signaling Pathway. Cancer Res. 2019;79:2257–70.
Li W, Cui K, Prochownik EV, Li Y. The deubiquitinase USP21 stabilizes MEK2 to promote tumor growth. Cell Death Dis. 2018;9:482.
Li Y, Cui K, Zhang Q, Li X, Lin X, Tang Y, et al. FBXL6 degrades phosphorylated p53 to promote tumor growth. Cell Death Differ. 2021;28:2112–25.
Zhu Y, Gu L, Lin X, Zhou X, Lu B, Liu C et al. P53 deficiency affects cholesterol esterification to exacerbate hepatocarcinogenesis. Hepatology. 2022. https://doi.org/10.1002/hep.32518.
Cao P, Yang A, Li P, Xia X, Han Y, Zhou G et al. Genomic gain of RRS1 promotes hepatocellular carcinoma through reducing the RPL11-MDM2-p53 signaling. Sci Adv. 2021;7:eabf4304.
Yu ZK, Geyer RK, Maki CG. MDM2-dependent ubiquitination of nuclear and cytoplasmic P53. Oncogene 2000;19:5892–7.
Shirangi TR, Zaika A, Moll UM. Nuclear degradation of p53 occurs during down-regulation of the p53 response after DNA damage. FASEB J. 2002;16:420–2.
Jiang T, Altman S. A protein subunit of human RNase P, Rpp14, and its interacting partner, OIP2, have 3’–>5’ exoribonuclease activity. Proc Natl Acad Sci. 2002;99:5295–300.
Lee HO, Hong Y, Etlioglu HE, Cho YB, Pomella V, Van den Bosch B, et al. Lineage-dependent gene expression programs influence the immune landscape of colorectal cancer. Nat Genet. 2020;52:594–603.
Hao Y, Hao S, Andersen-Nissen E, Mauck WM 3rd, Zheng S, Butler A, et al. Integrated analysis of multimodal single-cell data. Cell 2021;184:3573–87e3529.
Andreatta M, Carmona SJ. UCell: Robust and scalable single-cell gene signature scoring. Comput Struct Biotechnol J. 2021;19:3796–8.
Gao R, Bai S, Henderson YC, Lin Y, Schalck A, Yan Y, et al. Delineating copy number and clonal substructure in human tumors from single-cell transcriptomes. Nat Biotechnol. 2021;39:599–608.
Zhang J, Cui K, Huang L, Yang F, Sun S, Bian Z, et al. SLCO4A1-AS1 promotes colorectal tumourigenesis by regulating Cdk2/c-Myc signalling. J Biomed Sci. 2022;29:4.
Cui K, Yao S, Zhang H, Zhou M, Liu B, Cao Y, et al. Identification of an immune overdrive high-risk subpopulation with aberrant expression of FOXP3 and CTLA4 in colorectal cancer. Oncogene 2021;40:2130–45.
Gong L, Li Y, Cui K, Chen Y, Hong H, Li J, et al. Nanobody-Engineered Natural Killer Cell Conjugates for Solid Tumor Adoptive Immunotherapy. Small 2021;17:e2103463.
Bian Z, Zhou M, Cui K, Yang F, Cao Y, Sun S, et al. SNHG17 promotes colorectal tumorigenesis and metastasis via regulating Trim23-PES1 axis and miR-339-5p-FOSL2-SNHG17 positive feedback loop. J Exp Clin Cancer Res. 2021;40:360.
Gong Z, Li A, Ding J, Li Q, Zhang L, Li Y, et al. OTUD7B Deubiquitinates LSD1 to Govern Its Binding Partner Specificity, Homeostasis, and Breast Cancer Metastasis. Adv Sci (Weinh). 2021;8:e2004504.
Zhu Y, Gu L, Lin X, Liu C, Lu B, Cui K, et al. Dynamic Regulation of ME1 Phosphorylation and Acetylation Affects Lipid Metabolism and Colorectal Tumorigenesis. Mol Cell. 2020;77:138–49.
Hacot S, Coute Y, Belin S, Albaret MA, Mertani HC, Sanchez JC et al. Isolation of nucleoli. Curr. Protoc. Cell Biol. 2010;47:3.36.1–3.36.10.
Li D, Yao S, Zhou Z, Shi J, Huang Z, Wu Z. Hyaluronan decoration of milk exosomes directs tumor-specific delivery of doxorubicin. Carbohydr Res. 2020;493:108032.
Acknowledgements
We acknowledge the TCGA, GEO, CCLE and CPTAC projects. We would like to thank developers of each dataset, method and package used in this study. We thank the Affiliated Hospital of Jiangnan University for providing the CRC samples. We also thank the platforms of Medical Research Center (Wuxi School of Medicine, Jiangnan University). This work was supported by grants from the National Natural Science Foundation of China (82002550 and 82173063) and Wuxi Medical Key Discipline (ZDXK2021002).
Author information
Authors and Affiliations
Contributions
KC initiated the entire study. KC designed and performed the bioinformatics analyses and visualization. BL and QL provided support for bioinformatics analysis. KC, LG, ZG, YL, QZ and ZH designed the wet-lab experiments. KC, LG, HZ, and YW performed molecular biology-related experiments. KC, LG, HZ, YiC, and YuC conducted in vitro and in vivo experiments on proliferation phenotypes. KC and LG performed the ribosome biogenesis-related experiments. KC, LG, HZ, ZG and JL conducted protein-related assays. BL, SS, YiC, YuC and BF performed CRC samples collective and information maintenance. LG and BL conducted IHC assays. LG performed therapeutic experiments. KC, LG, BL, ZG and ZH designed graphical abstract, and KC visualized it. KC and ZH supervised this project and mentored the participants. KC wrote the manuscript. KC, LG, ZG and ZH critically revised the manuscript. All authors discussed the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, K., Gong, L., Zhang, H. et al. EXOSC8 promotes colorectal cancer tumorigenesis via regulating ribosome biogenesis-related processes. Oncogene 41, 5397–5410 (2022). https://doi.org/10.1038/s41388-022-02530-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02530-4