Abstract
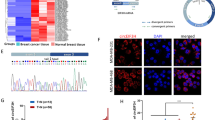
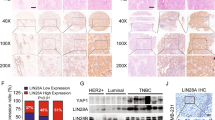
Emerging evidence shows that the lncRNA THOR is deeply involved in the development of various cancers. However, the effects and underlying molecular mechanisms of THOR in breast cancer (BRCA) initiation and progression have not been fully elucidated. Here we show that THOR is critical for BRCA tumorigenesis by interacting with hnRNPD to regulate downstream signaling pathways. THOR expression was significantly higher in BRCA tissues than in normal tissues, and THOR upregulation was associated with a poor prognosis in BRCA patients. Functionally, THOR knockdown impaired cell proliferation, migration and invasion in BRCA cells in vitro and inhibited tumorigenesis and metastasis in a tumor xenograft model and THOR-deficient MMTV-PyMT model in vivo. Mechanistically, THOR bound to the hnRNPD protein and increased hnRNPD protein levels by maintaining hnRNPD protein stability through inhibition of the proteasome-dependent degradation pathway. The increased hnRNPD protein levels led to stabilization of its target mRNAs, including pyruvate dehydrogenase kinase 1 (PDK1), further activating downstream PI3K–AKT and MAPK signaling pathways to regulate BRCA cell proliferation and metastasis. Together, our findings indicate that THOR is a promising prognostic predictor for BRCA patients and that the THOR–hnRNPD–PDK1–MAPK/PI3K–AKT axis might be a potential therapeutic target for BRCA treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71:209–49.
Qiu H, Cao S, Xu R. Cancer incidence, mortality, and burden in China: a time-trend analysis and comparison with the United States and United Kingdom based on the global epidemiological data released in 2020. Cancer Commun. 2021;41:1037–48.
Yi M, Li T, Niu M, Luo S, Chu Q, Wu K. Epidemiological trends of women’s cancers from 1990 to 2019 at the global, regional, and national levels: a population-based study. Biomark Res. 2021;9:55.
Ginsburg O, Bray F, Coleman MP, Vanderpuye V, Eniu A, Kotha SR, et al. The global burden of women’s cancers: a grand challenge in global health. Lancet. 2017;389:847–60.
Ulitsky I, Bartel DP. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154:26–46.
Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22:96–118.
Lin C, Yang L. Long noncoding RNA in cancer: wiring signaling circuitry. Trends Cell Biol. 2018;28:287–301.
Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev. 2016;96:1297–325.
Bartonicek N, Maag JL, Dinger ME. Long noncoding RNAs in cancer: mechanisms of action and technological advancements. Mol Cancer. 2016;15:43.
Liu SJ, Dang HX, Lim DA, Feng FY, Maher CA. Long noncoding RNAs in cancer metastasis. Nat Rev Cancer. 2021;21:446–60.
Schmitt AM, Chang HY. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29:452–63.
Amelio I, Bernassola F, Candi E. Emerging roles of long non-coding RNAs in breast cancer biology and management. Semin Cancer Biol. 2021;72:36–45.
St Laurent G, Wahlestedt C, Kapranov P. The Landscape of long noncoding RNA classification. Trends Genet. 2015;31:239–51.
Hosono Y, Niknafs YS, Prensner JR, Iyer MK, Dhanasekaran SM, Mehra R, et al. Oncogenic role of THOR, a conserved cancer/testis long non-coding RNA. Cell. 2017;171:1559–72.e1520.
Ulitsky I, Shkumatava A, Jan CH, Sive H, Bartel DP. Conserved function of lincRNAs in vertebrate embryonic development despite rapid sequence evolution. Cell. 2011;147:1537–50.
Tsagakis I, Douka K, Birds I, Aspden JL. Long non-coding RNAs in development anddisease: conservation to mechanisms. J Pathol. 2020;250:480–95.
Xue J, Zhong S, Sun BM, Sun QF, Hu LY, Pan SJ. Lnc-THOR silencing inhibits human glioma cell survival by activating MAGEA6-AMPK signaling. Cell Death Dis. 2019;10:866.
Yang H, Fu G, Liu F, Hu C, Lin J, Tan Z, et al. LncRNA THOR promotes tongue squamous cell carcinomas by stabilizing IGF2BP1 downstream targets. Biochimie. 2019;165:9–18.
Wang B, Ye Q, Zou C. Long non-coding RNA THOR enhances the stem cell-like traits of triple-negative breast cancer cells through activating beta-catenin signaling. Med Sci Monit: Int Med J Exp Clin Res. 2020;26:e923507.
Ge J, Han T, Shan L, Na J, Li Y, Wang J. Long non-coding RNA THOR promotes ovarian cancer cells progression via IL-6/STAT3 pathway. J Ovarian Res. 2020;13:72.
Wang SS, Lv Y, Xu XC, Zuo Y, Song Y, Wu GP, et al. Triptonide inhibits human nasopharyngeal carcinoma cell growth via disrupting Lnc-RNA THOR-IGF2BP1 signaling. Cancer Lett. 2019;443:13–24.
Song H, Xu Y, Shi L, Xu T, Fan R, Cao M, et al. LncRNA THOR increases the stemness of gastric cancer cells via enhancing SOX9 mRNA stability. Biomed Pharmacother. 2018;108:338–46.
Moore AE, Chenette DM, Larkin LC, Schneider RJ. Physiological networks and disease functions of RNA-binding protein AUF1. Wiley Interdiscip Rev RNA. 2014;5:549–64.
Li J, He M, Xu W, Huang S. LINC01354 interacting with hnRNP-D contributes to the proliferation and metastasis in colorectal cancer through activating Wnt/beta-catenin signaling pathway. J Exp Clin Cancer Res. 2019;38:161.
Ma W, Qiao J, Zhou J, Gu L, Deng D. Characterization of novel LncRNA P14AS as a protector of ANRIL through AUF1 binding in human cells. Mol Cancer. 2020;19:42.
Al-Khalaf HH, Aboussekhra A. MicroRNA-141 and microRNA-146b-5p inhibit the prometastatic mesenchymal characteristics through the RNA-binding protein AUF1 targeting the transcription factor ZEB1 and the protein kinase AKT. J Biol Chem. 2014;289:31433–47.
Gagliardi PA, Puliafito A, Primo L. PDK1: at the crossroad of cancer signaling pathways. Semin Cancer Biol. 2018;48:27–35.
Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21:1253–61.
Liang Y, Song X, Li Y, Chen B, Zhao W, Wang L, et al. LncRNA BCRT1 promotes breast cancer progression by targeting miR-1303/PTBP3 axis. Mol Cancer. 2020;19:85.
Zheng F, Chen J, Zhang X, Wang Z, Chen J, Lin X, et al. The HIF-1alpha antisense long non-coding RNA drives a positive feedback loop of HIF-1alpha mediated transactivation and glycolysis. Nat Commun. 2021;12:1341.
Ma H, Chang H, Yang W, Lu Y, Hu J, Jin S. A novel IFNalpha-induced long noncoding RNA negatively regulates immunosuppression by interrupting H3K27 acetylation in head and neck squamous cell carcinoma. Mol Cancer. 2020;19:4.
Wang Y, Chen W, Lian J, Zhang H, Yu B, Zhang M, et al. The lncRNA PVT1 regulates nasopharyngeal carcinoma cell proliferation via activating the KAT2A acetyltransferase and stabilizing HIF-1alpha. Cell Death Differ. 2020;27:695–710.
Hentze MW, Castello A, Schwarzl T, Preiss T. A brave new world of RNA-binding proteins. Nat Rev Mol Cell Biol. 2018;19:327–41.
Shang Y. LncRNA THOR acts as a retinoblastoma promoter through enhancing the combination of c-myc mRNA and IGF2BP1 protein. Biomed Pharmacother. 2018;106:1243–9.
Du X, Wang JM, Zhang DL, Wu T, Zeng XY, Jiang JY, et al. AUF1 promotes proliferation and invasion of thyroid cancer via downregulation of ZBTB2 and subsequent TRIM58. Front Oncol. 2021;11:681736.
Tsitsipatis D, Grammatikakis I, Driscoll RK, Yang X, Abdelmohsen K, Harris SC, et al. AUF1 ligand circPCNX reduces cell proliferation by competing with p21 mRNA to increase p21 production. Nucleic Acids Res. 2021;49:1631–46.
Mattijssen S, Kozlov G, Fonseca BD, Gehring K, Maraia RJ. LARP1 and LARP4: up close with PABP for mRNA 3’ poly(A) protection and stabilization. RNA Biol. 2021;18:259–74.
YuFeng Z, Ming Q. Expression and prognostic roles of PABPC1 in hepatocellular carcinoma. Int J Surg. 2020;84:3–12.
Feng X, Li J, Liu P. The biological roles of translation initiation factor 3b. Int J Biol Sci. 2018;14:1630–5.
Portz B, Lee BL, Shorter J. FUS and TDP-43 phases in health and disease. Trends Biochem Sci. 2021;46:550–63.
Fabbiano F, Corsi J, Gurrieri E, Trevisan C, Notarangelo M, D’Agostino VG. RNA packaging into extracellular vesicles: an orchestra of RNA-binding proteins? J Extracell Vesicles. 2020;10:e12043.
Yang MH, Zhao L, Wang L, Ou-Yang W, Hu SS, Li WL, et al. Nuclear lncRNA HOXD-AS1 suppresses colorectal carcinoma growth and metastasis via inhibiting HOXD3-induced integrin beta3 transcriptional activating and MAPK/AKT signalling. Mol Cancer. 2019;18:31.
Ding J, Zhao J, Huan L, Liu Y, Qiao Y, Wang Z, et al. Inflammation-induced long intergenic noncoding RNA (LINC00665) increases malignancy through activating the double-stranded RNA-activated protein kinase/nuclear factor kappa B pathway in hepatocellular carcinoma. Hepatology. 2020;72:1666–81.
Anwar S, Shamsi A, Mohammad T, Islam A, Hassan MI. Targeting pyruvate dehydrogenase kinase signaling in the development of effective cancer therapy. Biochim Biophys Acta Rev Cancer. 2021;1876:188568.
Zhang M, Cong Q, Zhang XY, Zhang MX, Lu YY, Xu CJ. Pyruvate dehydrogenase kinase 1 contributes to cisplatin resistance of ovarian cancer through EGFR activation. J Cell Physiol. 2019;234:6361–70.
Abdolahi S, Ghazvinian Z, Muhammadnejad S, Saleh M, Asadzadeh Aghdaei H, Baghaei K. Patient-derived xenograft (PDX) models, applications and challenges in cancer research. J Transl Med. 2022;20:206.
Wang Y, Wang W, Wu H, Zhou Y, Qin X, Wang Y, et al. The essential role of PRAK in tumor metastasis and its therapeutic potential. Nat Commun. 2021;12:1736.
Jin X, Ge LP, Li DQ, Shao ZM, Di GH, Xu XE, et al. LncRNA TROJAN promotes proliferation and resistance to CDK4/6 inhibitor via CDK2 transcriptional activation in ER+ breast cancer. Mol Cancer. 2020;19:87.
Acknowledgements
The authors would like to thank Xiaoguo Zheng and Chuanjin Yu at the International Peace Maternity and Child Health Hospital for their efforts in constructing THOR-KO cells by CRISPR–Cas9 technology. We thank Xin Ye at the International Peace Maternity and Child Health Hospital for helping collect clinical specimens. We also thank Rogier Versteeg and the Department of Oncogenomics at the Academic Medical Center (Amsterdam, The Netherlands) for providing the R2 Platform.
Funding
This work was supported by the National Natural Science Foundation of China (82088102, 82171686), National Key Research and Development Program of China (2021YFC2700701), Collaborative Innovation Program of Shanghai Municipal Health Commission (2020CXJQ01), CAMS Innovation Fund for Medical Sciences (2019-I2M-5-064), Clinical Research Plan of SHDC (SHDC2020CR1008A), Shanghai Municipal Key Clinical Specialty (shslczdzk06302), Program of Shanghai Academic Research Leader (20XD1424100), Outstanding Youth Medical Talents of Shanghai Rising Stars of Medical Talent Youth Development Program, and Interdisciplinary Program of Shanghai Jiao Tong University (ZH2018QNB15, YG2019GD04, YG2020YQ29), Youth Talents development Program of Jiangsu Women And Children Health Hospital (FYRC202004), and Young Scholars Fostering Fund of the First Affiliated Hospital of Nanjing Medical University (PY2021009). The funding agencies had no role in the study design, data collection and analysis, or decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
The study was conceived and designed by HFH, EWS, YTW, and XML. HTH and HQZ collected clinical specimens and data, conducted most of the experiments and analyzed the data. JW and QH collected clinical specimens. YX, YZ, CL, JJX and YYG performed some of the experiments. HTH wrote the manuscript. JJX, JW and QH provided crucial reagents and took part in discussions. HFH, YTW, JZS, and JNC revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Ethics approval for this study was granted by the Ethics Committee of the International Peace Maternity and Child Health Hospital, School of Medicine, Shanghai Jiao Tong University and Ethics Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University. All patients provided written informed consent before participating in this study. All animal experiments were approved by the Institutional Animal Care and Use Committee of GemPharmatech Co., Ltd. (Nanjing, China).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, H., Zhang, H., Xing, Y. et al. The lncRNA THOR interacts with and stabilizes hnRNPD to promote cell proliferation and metastasis in breast cancer. Oncogene 41, 5298–5314 (2022). https://doi.org/10.1038/s41388-022-02495-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02495-4