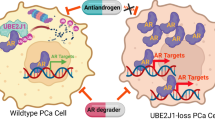
Abstract
Strategies to degrade steroid receptors and their alternative splicing isoforms are critical for disease management. Here we report that celastrol recruited the ubiquitin ligase UBE3A and degraded androgen receptor (AR), AR-v7, and glucocorticoid receptor (GR) to suppress prostate cancer development. UBE3A was not an optimal endogenous AR ubiquitin ligase in mice and patients, but celastrol promoted the interaction between UBE3A and AR. Multiple domains of AR, including the DNA binding domain (DBD), were implicated into the UBE3A-AR interaction. Sharing a conserved DBD, GR, AR-v7, and other steroid receptors were recognized and degraded by UBE3A after celastrol treatment. Thus, celastrol suppressed prostate cancer cell proliferation more potently than enzalutamide. Modifying the carboxyl group of celastrol improved its anti-tumor activity. Together, our findings revealed that celastrol might be a potential molecular glue to enhance the interaction between UBE3A and steroid receptors to degrade multiple steroid receptors and splicing isoforms in prostate cancer, paving a way for further drug optimization and disease treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data used and/or analysed during this study are available from the corresponding author on reasonable request.
References
Groner AC, Brown M. Role of steroid receptor and coregulator mutations in hormone-dependent cancers. J Clin Investig. 2017;127:1126–35.
Tata JR. Signalling through nuclear receptors. Nat Rev Mol Cell Biol. 2002;3:702–10.
Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol. 2008;8:440–8.
Furr BJ. The development of Casodex (bicalutamide): preclinical studies. Eur Urol. 1996;29:83–95. Suppl 2
Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl J Med. 2012;367:1187–97.
Beer TM, Armstrong AJ, Rathkopf DE, Loriot Y, Sternberg CN, Higano CS, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl J Med. 2014;371:424–33.
Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 2009;324:787–90.
Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl J Med. 2014;371:1028–38.
Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68:5469–77.
Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 2013;155:1309–22.
Efstathiou E, Titus M, Wen S, Hoang A, Karlou M, Ashe R, et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur Urol. 2015;67:53–60.
Li J, Berk M, Alyamani M, Sabharwal N, Goins C, Alvarado J, et al. Hexose-6-phosphate dehydrogenase blockade reverses prostate cancer drug resistance in xenograft models by glucocorticoid inactivation. Sci Translational Med. 2021;13:eabe8226.
Hou Z, Huang S, Li Z. Androgens in prostate cancer: A tale that never ends. Cancer Lett. 2021;516:1–12.
Schneekloth AR, Pucheault M, Tae HS, Crews CM. Targeted intracellular protein degradation induced by a small molecule: En route to chemical proteomics. Bioorg Med Chem Lett. 2008;18:5904–8.
Toure M, Crews CM. Small-Molecule PROTACS: New Approaches to Protein Degradation. Angew Chem. 2016;55:1966–73.
Takwale AD, Jo SH, Jeon YU, Kim HS, Shin CH, Lee HK, et al. Design and characterization of cereblon-mediated androgen receptor proteolysis-targeting chimeras. Eur J Med Chem. 2020;208:112769.
Zhao L, Han X, Lu J, McEachern D, Wang S. A highly potent PROTAC androgen receptor (AR) degrader ARD-61 effectively inhibits AR-positive breast cancer cell growth in vitro and tumor growth in vivo. Neoplasia 2020;22:522–32.
Han X, Wang C, Qin C, Xiang W, Fernandez-Salas E, Yang CY, et al. Discovery of ARD-69 as a Highly Potent Proteolysis Targeting Chimera (PROTAC) Degrader of Androgen Receptor (AR) for the Treatment of Prostate Cancer. J Med Chem. 2019;62:941–64.
Alyamani M, Li Z, Berk M, Li J, Tang J, Upadhyay S, et al. Steroidogenic Metabolism of Galeterone Reveals a Diversity of Biochemical Activities. Cell Chem Biol. 2017;24:825–32. e6
Ponnusamy S, He Y, Hwang DJ, Thiyagarajan T, Houtman R, Bocharova V, et al. Orally Bioavailable Androgen Receptor Degrader, Potential Next-Generation Therapeutic for Enzalutamide-Resistant Prostate Cancer. Clin Cancer Res: Off J Am Assoc Cancer Res. 2019;25:6764–80.
Yang Z, Chang YJ, Yu IC, Yeh S, Wu CC, Miyamoto H, et al. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat Med. 2007;13:348–53.
Hieronymus H, Lamb J, Ross KN, Peng XP, Clement C, Rodina A, et al. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer cell. 2006;10:321–30.
Yang H, Chen D, Cui QC, Yuan X, Dou QP. Celastrol, a triterpene extracted from the Chinese “Thunder of God Vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006;66:4758–65.
Chadli A, Felts SJ, Wang Q, Sullivan WP, Botuyan MV, Fauq A, et al. Celastrol inhibits Hsp90 chaperoning of steroid receptors by inducing fibrillization of the Co-chaperone p23. J Biol Chem 2010;285:4224–31.
Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993;75:495–505.
Huibregtse JM, Scheffner M, Howley PM. Cloning and expression of the cDNA for E6-AP, a protein that mediates the interaction of the human papillomavirus E6 oncoprotein with p53. Mol Cell Biol 1993;13:775–84.
Fang M, Li Y, Ren J, Hu R, Gao X, Chen L. Epilepsy-Associated UBE3A Deficiency Downregulates Retinoic Acid Signalling Pathway. Front Genet. 2021;12:681295.
Nawaz Z, Lonard DM, Smith CL, Lev-Lehman E, Tsai SY, Tsai MJ, et al. The Angelman syndrome-associated protein, E6-AP, is a coactivator for the nuclear hormone receptor superfamily. Mol Cell Biol. 1999;19:1182–9.
Khan OY, Fu G, Ismail A, Srinivasan S, Cao X, Tu Y, et al. Multifunction steroid receptor coactivator, E6-associated protein, is involved in development of the prostate gland. Mol Endocrinol. 2006;20:544–59.
Birch SE, Kench JG, Takano E, Chan P, Chan AL, Chiam K, et al. Expression of E6AP and PML predicts for prostate cancer progression and cancer-specific death. Ann Oncol: Off J Eur Soc Med Oncol. 2014;25:2392–7.
Smith CL, DeVera DG, Lamb DJ, Nawaz Z, Jiang YH, Beaudet AL, et al. Genetic ablation of the steroid receptor coactivator-ubiquitin ligase, E6-AP, results in tissue-selective steroid hormone resistance and defects in reproduction. Mol Cell Biol. 2002;22:525–35.
Jones JO, An WF, Diamond MI. AR inhibitors identified by high-throughput microscopy detection of conformational change and subcellular localization. ACS Chem Biol. 2009;4:199–208.
Li H, Ban F, Dalal K, Leblanc E, Frewin K, Ma D, et al. Discovery of small-molecule inhibitors selectively targeting the DNA-binding domain of the human androgen receptor. J Med Chem. 2014;57:6458–67.
Zhou Y, Li W, Wang M, Zhang X, Zhang H, Tong X, et al. Competitive profiling of celastrol targets in human cervical cancer HeLa cells via quantitative chemical proteomics. Mol Biosyst. 2016;13:83–91.
Salminen A, Lehtonen M, Paimela T, Kaarniranta K. Celastrol: Molecular targets of Thunder God Vine. Biochemical Biophysical Res Commun. 2010;394:439–42.
Lee JH, Koo TH, Yoon H, Jung HS, Jin HZ, Lee K, et al. Inhibition of NF-kappa B activation through targeting I kappa B kinase by celastrol, a quinone methide triterpenoid. Biochemical Pharmacol. 2006;72:1311–21.
Seo HR, Seo WD, Pyun BJ, Lee BW, Jin YB, Park KH, et al. Radiosensitization by celastrol is mediated by modification of antioxidant thiol molecules. Chem-Biol Interact. 2011;193:34–42.
Hou Z, Huang S, Mei Z, Chen L, Guo J, Gao Y, et al. Inhibiting 3betaHSD1 to eliminate the oncogenic effects of progesterone in prostate cancer. Cell Rep Med. 2022;3:100561.
Mei Z, Yang T, Liu Y, Gao Y, Hou Z, Zhuang Q, et al. Management of prostate cancer by targeting 3betaHSD1 after enzalutamide and abiraterone treatment. Cell Rep Med. 2022;3:100608.
Kregel S, Wang C, Han X, Xiao L, Fernandez-Salas E, Bawa P, et al. Androgen receptor degraders overcome common resistance mechanisms developed during prostate cancer treatment. Neoplasia 2020;22:111–9.
Proof-of-Concept with PROTACs in Prostate Cancer. Cancer Discov. 2020;10:1084. https://doi.org/10.1158/2159-8290.CD-NB2020-054.
Lim HY, Ong PS, Wang L, Goel A, Ding L, Li-Ann Wong A, et al. Celastrol in cancer therapy: Recent developments, challenges and prospects. Cancer Lett. 2021;521:252–67.
Shi J, Li J, Xu Z, Chen L, Luo R, Zhang C, et al. Celastrol: A Review of Useful Strategies Overcoming its Limitation in Anticancer Application. Front Pharmacol. 2020;11:558741.
Ramamoorthy S, Nawaz Z. E6-associated protein (E6-AP) is a dual function coactivator of steroid hormone receptors. Nucl Receptor Signal. 2008;6:e006.
Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat Genet. 1997;15:70–3.
Matsuura T, Sutcliffe JS, Fang P, Galjaard RJ, Jiang YH, Benton CS, et al. De novo truncating mutations in E6-AP ubiquitin-protein ligase gene (UBE3A) in Angelman syndrome. Nat Genet. 1997;15:74–7.
Gao X, Mohsin SK, Gatalica Z, Fu G, Sharma P, Nawaz Z. Decreased expression of e6-associated protein in breast and prostate carcinomas. Endocrinology 2005;146:1707–12.
Jiang YH, Armstrong D, Albrecht U, Atkins CM, Noebels JL, Eichele G, et al. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron 1998;21:799–811.
Park JH, Walls JE, Galvez JJ, Kim M, Abate-Shen C, Shen MM, et al. Prostatic intraepithelial neoplasia in genetically engineered mice. Am J Pathol. 2002;161:727–35.
Shaffer PL, Jivan A, Dollins DE, Claessens F, Gewirth DT. Structural basis of androgen receptor binding to selective androgen response elements. Proc Natl Acad Sci. 2004;101:4758–63.
Xu X, Li C, Gao X, Xia K, Guo H, Li Y, et al. Excessive UBE3A dosage impairs retinoic acid signaling and synaptic plasticity in autism spectrum disorders. Cell Res. 2018;28:48–68.
Acknowledgements
We thank the staff members (Zhuo Yang, et al) of the chemical biology core facility, the high-performance computing service platform, and the bioinformatics core in Shanghai Institute of Biochemistry and Cell Biology (SIBCB), mass spectrometry staff members of the National Facility for Protein in Shanghai (NFPS), and the Zhangjiang Lab and staff members (Pengyu Wang, et al.) of Bio-Med Big Data Center /Shanghai Institute of Nutrition and Health (SINH) for providing technical support and assistance in data collection and analysis. This work was supported by funding from the National Key R&D Program of China (2018YFA0508200 to ZL; 2019YFA0802103 and 2018YFA0508200 to RH), National Natural Science Foundation of China (92157101 to ZL; 81973166, 91753207 to BZ; 91853128, 81525019 to RH). RH was also supported by funding from Department of Science and Technology of Zhejiang Province (Proj. No. 2021C03104).
Author information
Authors and Affiliations
Contributions
ZL initiated the project. ZL, RH and BZ designed the studies. QT, ZQL, XG and JC conducted functional assay. BZ, YW and LL synthesized celastrol related probes and compounds. XQ, HG, DW and SH were responsible for clinical data. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tan, Q., Liu, Z., Gao, X. et al. Celastrol recruits UBE3A to recognize and degrade the DNA binding domain of steroid receptors. Oncogene 41, 4754–4767 (2022). https://doi.org/10.1038/s41388-022-02467-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02467-8