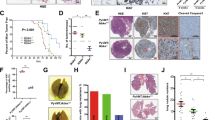
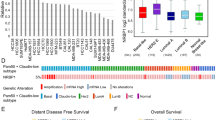
Abstract
Most basal-like breast cancers (BLBCs) are triple-negative breast cancers (TNBCs), which is associated with high malignancy, high rate of recurrence and distant metastasis, and poor prognosis among all types of breast cancer. However, there are currently no effective therapies for BLBC. Furthermore, chemoresistance limits the therapeutic options for BLBC treatment. In this study, we screen out protein activator of the interferon-induced protein kinase (PACT) as an essential gene in BLBC metastasis. We find that high PACT expression level was associated with poor prognosis among BLBC patients. In vivo and in vitro investigations indicated that PACT could regulate BLBC metastasis by interacting with SUMO-conjugating enzyme Ubc9 to stimulate the SUMOylation and thus consequently the activation of Rac1. BLBC patients receiving chemotherapy presents poorer prognosis with PACT high expression, and PACT disruption sensitizes experimental mammary tumor metastases to chemotherapy, thus providing insights to consider PACT as a potential therapeutic target to overcome acquired chemoresistance in BLBC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
RNA seq and RIP seq data generated for this publication have been deposited in NCBI’s SRA database under the accession: PRJNA643693
References
Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn H-J. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2009. Ann Oncol. 2009;20:1319–29.
Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–52.
Jiang Y-Z, Ma D, Suo C, Shi J, Xue M, Hu X, et al. Genomic and transcriptomic landscape of triple-negative breast cancers: subtypes and treatment strategies. Cancer Cell. 2019;35:428–40.
Polyak K. Heterogeneity in breast cancer. J Clin Invest. 2011;121:3786–8.
Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68.
Rios AC, Capaldo BD, Vaillant F, Pal B, van Ineveld R, Dawson CA, et al. Intraclonal plasticity in mammary tumors revealed through large-scale single-cell resolution 3D Imaging. Cancer Cell. 2019;35:618–32.
Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 2001;98:10869–74.
Voduc KD, Cheang MCU, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. JCO. 2010;28:1684–91.
Patel RC. PACT, a protein activator of the interferon-induced protein kinase, PKR. EMBO J. 1998;17:4379–90.
Bennett RL, Blalock WL, Abtahi DM, Pan Y, Moyer SA, May WS. RAX, the PKR activator, sensitizes cells to inflammatory cytokines, serum withdrawal, chemotherapy, and viral infection. Blood. 2006;108:821–9.
Huang X, Hutchins B, Patel RC. The C-terminal, third conserved motif of the protein activator PACT plays an essential role in the activation of double-stranded-RNA-dependent protein kinase (PKR). Biochemical J. 2002;366:175–86.
Bennett R, Pan Y, Christian J, Hui T, May WS Jr. The RAX/PACT-PKR stress response pathway promotes p53 sumoylation and activation, leading to G 1 arrest. Cell Cycle. 2012;11:407–17.
Bennett RL, Blalock WL, Choi E-J, Lee YJ, Zhang Y, Zhou L, et al. RAX is required for fly neuronal development and mouse embryogenesis. Mechanisms Dev. 2008;125:777–85.
Peters GA, Seachrist DD, Keri RA, Sen GC. The double-stranded RNA-binding protein, PACT, is required for postnatal anterior pituitary proliferation. Proc Natl Acad Sci. 2009;106:10696–701.
Rowe TM, Rizzi M, Hirose K, Peters GA, Sen GC. A role of the double-stranded RNA-binding protein PACT in mouse ear development and hearing. Proc Natl Acad Sci USA 2006;103:5823–8.
Yong Y, Meng Y, Ding H, Fan Z, Tang Y, Zhou C, et al. PACT/RAX regulates the migration of cerebellar granule neurons in the developing cerebellum. Sci Rep. 2015;5:7961.
Chiosea S, Acquafondata M, Luo J, Kuan SF, Seethala RR. DICER1 and PRKRA in colon adenocarcinoma. Biomark Insights. 2008;3:253–8.
Hisamatsu T, McGuire M, Wu SY, Rupaimoole R, Pradeep S, Bayraktar E, et al. PRKRA/PACT expression promotes chemoresistance of mucinous ovarian cancer. Mol Cancer Ther. 2019;18:162–72.
Mullany LE, Herrick JS, Wolff RK, Buas MF, Slattery ML. Impact of polymorphisms in microRNA biogenesis genes on colon cancer risk and microRNA expression levels: a population-based, case-control study. BMC Med Genomics. 2016;9:21.
Hay RT. Protein modification by SUMO. Trends Biochemical Sci. 2001;26:332–3.
Melchior F. SUMO—nonclassical ubiquitin. Annu Rev Cell Dev Biol. 2000;16:591–626.
Bernier-Villamor V, Sampson DA, Matunis MJ, Lima CD. Structural basis for E2-mediated SUMO conjugation revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and RanGAP1. Cell. 2002;108:345–56.
Liu Y, Zhao D, Qiu F, Zhang L-L, Liu S-K, Li Y-Y, et al. Manipulating PML SUMOylation via silencing UBC9 and RNF4 regulates cardiac fibrosis. Mol Ther. 2017;25:666–78.
Schwartz S, Truglio M, Scott MJ, Fitzsimons HL. Long-term memory in Drosophila is influenced by histone deacetylase HDAC4 interacting with SUMO-conjugating enzyme Ubc9. Genetics 2016;203:1249–64.
Ho C-W, Chen H-T, Hwang J. UBC9 autosumoylation negatively regulates sumoylation of septins in Saccharomyces cerevisiae. J Biol Chem. 2011;286:21826–34.
Su Y-F, Yang T, Huang H, Liu LF, Hwang J. Phosphorylation of Ubc9 by Cdk1 enhances SUMOylation activity. PLoS One. 2012;7:e34250.
Dünnebier T, Bermejo JL, Haas S, Fischer H-P, Pierl CB, Justenhoven C, et al. Polymorphisms in the UBC9 and PIAS3 genes of the SUMO-conjugating system and breast cancer risk. Breast Cancer Res Treat. 2010;121:185–94.
Li H, Niu H, Peng Y, Wang J, He P. Ubc9 promotes invasion and metastasis of lung cancer cells. Oncol Rep. 2013;29:1588–94.
Xu J, Footman A, Qin Y, Aysola K, Black S, Reddy V, et al. BRCA1 mutation leads to deregulated ubc9 levels which triggers proliferation and migration of patient-derived high grade serous ovarian cancer and triple negative breast cancer cells. Int J Chronic Dis Ther. 2016;2:31–8.
Kheradmand F. Role of Rac1 and oxygen radicals in collagenase-1 expression induced by cell shape change. Science. 1998;280:898–902.
Bristow JM, Sellers MH, Majumdar D, Anderson B, Hu L, Webb DJ. The Rho-family GEF Asef2 activates Rac to modulate adhesion and actin dynamics and thereby regulate cell migration. J Cell Sci. 2009;122:4535–46.
Chen H-Y, Shen C-H, Tsai Y-T, Lin F-C, Huang Y-P, Chen R-H. Brk activates Rac1 and promotes cell migration and invasion by phosphorylating paxillin. MCB. 2004;24:10558–72.
Castillo-Lluva S, Tatham MH, Jones RC, Jaffray EG, Edmondson RD, Hay RT, et al. SUMOylation of the GTPase Rac1 is required for optimal cell migration. Nat Cell Biol. 2010;12:1078–85.
Lorente M, García-Casas A, Salvador N, Martínez-López A, Gabicagogeascoa E, Velasco G, et al. Inhibiting SUMO1-mediated SUMOylation induces autophagy-mediated cancer cell death and reduces tumour cell invasion via RAC1. J Cell Sci. 2019;132:jcs234120.
Kamai T. Overexpression of RhoA, Rac1, and Cdc42 GTPases is associated with progression in testicular cancer. Clin Cancer Res. 2004;10:4799–805.
Pan Y, Bi F, Liu N, Xue Y, Yao X, Zheng Y, et al. Expression of seven main Rho family members in gastric carcinoma. Biochemical Biophysical Res Commun. 2004;315:686–91.
Schnelzer A, Prechtel D, Knaus U, Dehne K, Gerhard M, Graeff H, et al. Rac1 in human breast cancer: overexpression, mutation analysis, and characterization of a new isoform, Rac1b. Oncogene. 2000;19:3013–20.
Mack NA, Whalley HJ, Castillo-Lluva S, Malliri A. The diverse roles of Rac signaling in tumorigenesis. Cell Cycle. 2011;10:1571–81.
Li Q, Qin T, Bi Z, Hong H, Ding L, Chen J, et al. Rac1 activates non-oxidative pentose phosphate pathway to induce chemoresistance of breast cancer. Nat Commun. 2020;11:1456.
Cannon AC, Uribe-Alvarez C, Chernoff J. RAC1 as a therapeutic target in malignant melanoma. Trends Cancer. 2020;6:478–88.
Marei H, Malliri A. Rac1 in human diseases: the therapeutic potential of targeting Rac1 signaling regulatory mechanisms. Small GTPases. 2017;8:139–63. 03
Doench JG, Fusi N, Sullender M, Hegde M, Vaimberg EW, Donovan KF, et al. Optimized sgRNA design to maximize activity and minimize off-target effects of CRISPR-Cas9. Nat Biotechnol. 2016;34:184–91.
Meyers RM, Bryan JG, McFarland JM, Weir BA, Sizemore AE, Xu H, et al. Computational correction of copy number effect improves specificity of CRISPR–Cas9 essentiality screens in cancer cells. Nat Genet. 2017;49:1779–84.
Tsherniak A, Vazquez F, Montgomery PG, Weir BA, Kryukov G, Cowley GS, et al. Defining a cancer dependency map. Cell. 2017;170:564–76.
DepMap, Broad. DepMap 21Q3 Public. figshare; 2021. https://figshare.com/articles/dataset/DepMap_21Q3_Public/15160110/2.
Jin X, Demere Z, Nair K, Ali A, Ferraro GB, Natoli T, et al. A metastasis map of human cancer cell lines. Nature. 2020;588:331–6.
The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70.
Fritsch R, de Krijger I, Fritsch K, George R, Reason B, Kumar MS, et al. RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell. 2013;153:1050–63.
García MA, Carrasco E, Aguilera M, Alvarez P, Rivas C, Campos JM, et al. The Chemotherapeutic Drug 5-Fluorouracil Promotes PKR-Mediated Apoptosis in a p53- Independent Manner in Colon and Breast Cancer Cells. PLoS ONE. 2011;6:e23887.
Peidis P, Papadakis AI, Muaddi H, Richard S, Koromilas AE. Doxorubicin bypasses the cytoprotective effects of eIF2α phosphorylation and promotes PKR-mediated cell death. Cell Death Differ. 2011;18:145–54.
Yoon CH, Lee ES, Lim DS, Bae YS. PKR, a p53 target gene, plays a crucial role in the tumor-suppressor function of p53. Proc Natl Acad Sci. 2009;106:7852–7.
Dickerman BK, White CL, Kessler PM, Sadler AJ, Williams BRG, Sen GC. The protein activator of protein kinase R, PACT/RAX, negatively regulates protein kinase R during mouse anterior pituitary development. FEBS J. 2015;282:4766–81.
Yoon C, Cho S-J, Chang KK, Park DJ, Ryeom SW, Yoon SS. Role of Rac1 pathway in epithelial-to-mesenchymal transition and cancer stem-like cell phenotypes in gastric adenocarcinoma. Mol Cancer Res. 2017;15:1106–16.
Dokmanovic M, Hirsch DS, Shen Y, Wu WJ. Rac1 contributes to trastuzumab resistance of breast cancer cells: Rac1 as a potential therapeutic target for the treatment of trastuzumab-resistant breast cancer. Mol Cancer Ther. 2009;8:1557–69.
Reijnders MRF, Ansor NM, Kousi M, Yue WW, Tan PL, Clarkson K, et al. RAC1 missense mutations in developmental disorders with diverse phenotypes. Am J Hum Genet. 2017;101:466–77.
Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–10.
Vincent S, Settleman J. The PRK2 kinase is a potential effector target of both Rho and Rac GTPases and regulates actin cytoskeletal organization. Mol Cell Biol. 1997;17:2247–56.
Svensmark JH, Brakebusch C. Rho GTPases in cancer: friend or foe? Oncogene. 2019;38:7447–56.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005;102:15545–50.
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–102.
Acknowledgements
This research was supported by the National Natural Science Foundation of China (31701217 to LW, and 31570921 to ZJK), Shanghai Key Laboratory of Health Identification and Assessment (13DZ2261000), Shanghai Municipal Commission of Health and Family Planning (201540206 to ZJK).
Author information
Authors and Affiliations
Contributions
ZJK and JL oversaw the study. ZJK and LW designed the research, outlined the manuscript, contributed to the literature search. ZJK, DW and XW contribute to manuscript editing. LW, WW, ZC and ZX contribute to data collection, and data analysis, provided the figures. LW drafted the manuscript, helped with data interpretation with additional input from all authors. JY collected clinical sample and did the staining.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
All animal experiments were carried out with the approval of the institutional Animal Care and Use Committee at Shanghai University of Traditional Chinese Medicine. (Ethics license, PZSHUTCM191108010). The breast cancer specimens used in the current study were obtained from The Zhongshan Hospital of Fudan University, Qingpu branch with patient consent and institutional review board approval.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, L., Wang, W., Yao, J. et al. PACT promotes the metastasis of basal-like breast cancer through Rac1 SUMOylation and activation. Oncogene 41, 4282–4294 (2022). https://doi.org/10.1038/s41388-022-02431-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02431-6
This article is cited by
-
Visualization of breast cancer-related protein synthesis from the perspective of bibliometric analysis
European Journal of Medical Research (2023)