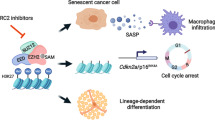
Abstract
Transcription dysregulation is a salient characteristic of bladder cancer (BC), but no appropriate therapeutic target for it has been established. Here, we found that heterogeneous downregulation of histone H4 transcription factor (HINFP) was associated with senescence in BC tissues and that lower HINFP expression could predict an unfavorable outcome in BC patients. Knockout of HINFP transcriptionally inhibited H1F0 and H1FX to trigger DNA damage, consequently inducing cell senescence to repress the proliferation and growth of BC cells. However, the senescence-associated secretory phenotype, characterized by increases in MMP1/3, enhances the invasion and metastasis of non-senescent BC cells. Histone deacetylase inhibitors (HDACis) could efficiently eliminate the senescent cells induced by HINFP knockout to suppress the invasion and metastasis of BC cells. Our study suggests that HDACis, widely used in multiple cancer types in a clinical context, may also benefit BC patients with metastases induced by cell senescence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The RNA-Seq data were deposited in the Genome Sequence Archive for Humans with accession code HRA001354. Other data used for the current study are available from the corresponding author upon reasonable request.
References
Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmstrom PU, Choi W, et al. Bladder cancer. Lancet. 2016;388:2796–810.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30.
Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur Urol. 2017;71:96–108.
von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8.
Stein JP, Skinner DG. Radical cystectomy for invasive bladder cancer: long-term results of a standard procedure. World J Urol. 2006;24:296–304.
Guo Y, Yuan X, Li K, Dai M, Zhang L, Wu Y, et al. GABPA is a master regulator of luminal identity and restrains aggressive diseases in bladder cancer. Cell Death Differ. 2020;27:1862–77.
Schmitz-Drager BJ, Schulz WA, Jurgens B, Gerharz CD, van Roeyen CR, Bultel H, et al. c-myc in bladder cancer. Clinical findings and analysis of mechanism. Urol Res. 1997;25:S45–49.
Zhang Z, Xie D, Li X, Wong YC, Xin D, Guan XY, et al. Significance of TWIST expression and its association with E-cadherin in bladder cancer. Hum Pathol. 2007;38:598–606.
Hayden A, Douglas J, Sommerlad M, Andrews L, Gould K, Hussain S, et al. The Nrf2 transcription factor contributes to resistance to cisplatin in bladder cancer. Urol Oncol. 2014;32:806–14.
Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci USA. 2009;106:14016–21.
Koike-Yusa H, Li Y, Tan EP, Velasco-Herrera Mdel C, Yusa K. Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nat Biotechnol. 2014;32:267–73.
Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–7.
Zhou Y, Zhu S, Cai C, Yuan P, Li C, Huang Y, et al. High-throughput screening of a CRISPR/Cas9 library for functional genomics in human cells. Nature. 2014;509:487–91.
Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science. 2014;343:80–4.
Wang T, Birsoy K, Hughes NW, Krupczak KM, Post Y, Wei JJ, et al. Identification and characterization of essential genes in the human genome. Science. 2015;350:1096–101.
Hart T, Chandrashekhar M, Aregger M, Steinhart Z, Brown KR, MacLeod G, et al. High-Resolution CRISPR Screens Reveal Fitness Genes and Genotype-Specific Cancer Liabilities. Cell. 2015;163:1515–26.
Faget DV, Ren Q, Stewart SA. Unmasking senescence: context-dependent effects of SASP in cancer. Nat Rev Cancer. 2019;19:439–53.
Demaria M, O’Leary MN, Chang J, Shao L, Liu S, Alimirah F, et al. Cellular Senescence Promotes Adverse Effects of Chemotherapy and Cancer Relapse. Cancer Discov. 2017;7:165–76.
Sulli G, Di Micco R, d’Adda di Fagagna F. Crosstalk between chromatin state and DNA damage response in cellular senescence and cancer. Nat Rev Cancer. 2012;12:709–20.
Funayama R, Saito M, Tanobe H, Ishikawa F. Loss of linker histone H1 in cellular senescence. J Cell Biol. 2006;175:869–80.
Milanovic M, Yu Y, Schmitt CA. The Senescence-Stemness Alliance—A Cancer-Hijacked Regeneration Principle. Trends Cell Biol. 2018;28:1049–61.
Kim YH, Choi YW, Lee J, Soh EY, Kim JH, Park TJ. Senescent tumor cells lead the collective invasion in thyroid cancer. Nat Commun. 2017;8:15208.
Guccini I, Revandkar A, D’Ambrosio M, Colucci M, Pasquini E, Mosole S, et al. Senescence Reprogramming by TIMP1 Deficiency Promotes Prostate Cancer Metastasis. Cancer Cell. 2021;39:68–82. e69
Brooks TA, Hurley LH. The role of supercoiling in transcriptional control of MYC and its importance in molecular therapeutics. Nat Rev Cancer. 2009;9:849–61.
Valencia AM, Kadoch C. Chromatin regulatory mechanisms and therapeutic opportunities in cancer. Nat Cell Biol. 2019;21:152–61.
Fane M, Weeraratna AT. How the ageing microenvironment influences tumour progression. Nat Rev Cancer. 2020;20:89–106.
Li C, Cantor WJ, Nili N, Robinson R, Fenkell L, Tran YL, et al. Arterial repair after stenting and the effects of GM6001, a matrix metalloproteinase inhibitor. J Am Coll Cardiol. 2002;39:1852–8.
Wang L, Leite de Oliveira R, Huijberts S, Bosdriesz E, Pencheva N, Brunen D, et al. An Acquired Vulnerability of Drug-Resistant Melanoma with Therapeutic Potential. Cell. 2018;173:1413–25.
Sardi I, Dal Canto M, Bartoletti R, Guazzelli R, Travaglini F, Montali E. Molecular genetic alterations of c-myc oncogene in superficial and locally advanced bladder cancer. Eur Urol. 1998;33:424–30.
Xie R, Medina R, Zhang Y, Hussain S, Colby J, Ghule P, et al. The histone gene activator HINFP is a nonredundant cyclin E/CDK2 effector during early embryonic cell cycles. Proc Natl Acad Sci USA. 2009;106:12359–64.
Ghule PN, Xie RL, Colby JL, Rivera-Perez JA, Jones SN, Lian JB, et al. Maternal expression and early induction of histone gene transcription factor Hinfp sustains development in pre-implantation embryos. Dev Biol. 2016;419:311–20.
Liu LJ, Xie R, Hussain S, Lian JB, Rivera-Perez J, Jones SN, et al. Functional coupling of transcription factor HiNF-P and histone H4 gene expression during pre- and post-natal mouse development. Gene. 2011;483:1–10.
Sun Y, Coppe JP, Lam EW. Cellular Senescence: The Sought or the Unwanted? Trends Mol Med. 2018;24:871–85.
Sieben CJ, Sturmlechner I, van de Sluis B, van Deursen JM. Two-Step Senescence-Focused Cancer Therapies. Trends Cell Biol. 2018;28:723–37.
Watanabe S, Kawamoto S, Ohtani N, Hara E. Impact of senescence-associated secretory phenotype and its potential as a therapeutic target for senescence-associated diseases. Cancer Sci. 2017;108:563–9.
Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83.
Cai Y, Zhou H, Zhu Y, Sun Q, Ji Y, Xue A, et al. Elimination of senescent cells by beta-galactosidase-targeted prodrug attenuates inflammation and restores physical function in aged mice. Cell Res. 2020;30:574–89.
Xu Y, Zhang P, Liu Y. Chidamide tablets: HDAC inhibition to treat lymphoma. Drugs Today. 2017;53:167–76.
Acknowledgements
We thank Dr. Meisongzhu Yang from SYSUCC for her help in constructing the animal models and Dr. Jietian Jin from SYSUCC for help with pathological diagnosis. This work was supported by grants from the National Key Research and Development Program of China (2021YFA1300601 to TK, 2020YFA0509400 to JP), the National Nature Science Foundation of China (NSFC) (82030090 to TK, 82073103 to ZWL, 82103264 to XCZ, 82172939 to YW, 82002917 to LWZ), and the Science and Technology Program of Guangzhou (202002020092).
Author information
Authors and Affiliations
Contributions
XZ, YW, ZWL, and TK conceived the project, designed the experiments, and wrote the manuscript. XZ assisted by YW and ZFL performed most of the experiments and analyzed the data. ZL provided BC samples. JZ, LWZ, CJ, LSZ, RZ, and JP assisted with the experiments and provided technical assistance. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zheng, X., Liu, Z., Zhong, J. et al. Downregulation of HINFP induces senescence-associated secretory phenotype to promote metastasis in a non-cell-autonomous manner in bladder cancer. Oncogene 41, 3587–3598 (2022). https://doi.org/10.1038/s41388-022-02371-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02371-1