Abstract
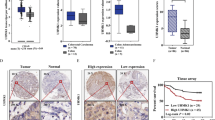
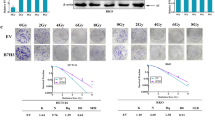
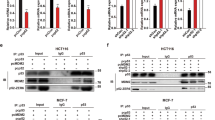
RIO Kinase 1 (RIOK1) is involved in various pathologies, including cancer. However, the role of RIOK1 in radioresistance of colorectal cancer (CRC) remains largely unknown. In this study, we reported that RIOK1 was overexpressed in rectal cancer tissue with weaker tumor regression after neoadjuvant chemoradiotherapy (neoCRT). Moreover, higher RIOK1 expression predicted a poor prognosis in patients with rectal cancer. Blockade of RIOK1 using Toyocamycin, a pharmacological inhibitor of RIOK1, or by knocking down its expression, decreased the resistance of CRC cells to radiotherapy in vitro and in vivo. A mechanistic study revealed that RIOK1 regulates radioresistance by suppressing the p53 signaling pathway. Furthermore, we found that RIOK1 and Ras-GAP SH3 domain binding protein 2 (G3BP2) interact with each other. RIOK1 phosphorylates G3BP2 at Thr226, which increases the activity of G3BP2. RIOK1-mediated phosphorylation of G3BP2 facilitated ubiquitination of p53 by murine double minute 2 protein (MDM2). Altogether, our study revealed the clinical significance of RIOK1 in CRC, and therapies targeting RIOK1 might alleviate the CRC tumor burden in patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The RNA-seq datasets analyzed for this study are available in the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/sra/). BioProject accession number: PRJNA827462.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Gu X, Zheng R, Xia C, Zeng H, Zhang S, Zou X, et al. Interactions between life expectancy and the incidence and mortality rates of cancer in China: a population-based cluster analysis. Cancer Commun. 2018;38:44.
Baker B, Salameh H, Al-Salman M, Daoud F. How does preoperative radiotherapy affect the rate of sphincter-sparing surgery in rectal cancer? Surg Oncol. 2012;21:e103–9.
Yang P, Yang Y, An W, Xu J, Zhang G, Jie J, et al. The long noncoding RNA-ROR promotes the resistance of radiotherapy for human colorectal cancer cells by targeting the p53/miR-145 pathway. J Gastroenterol Hepatol. 2017;32:837–45.
Kim BM, Hong Y, Lee S, Liu P, Lim JH, Lee YH, et al. Therapeutic implications for overcoming radiation resistance in cancer therapy. Int J Mol Sci. 2015;16:26880–913.
O’Connor MJ. Targeting the DNA damage response in cancer. Mol Cell. 2015;60:547–60.
Hu S, Cao B, Zhang M, Linghu E, Zhan Q, Brock MV, et al. Epigenetic silencing BCL6B induced colorectal cancer proliferation and metastasis by inhibiting P53 signaling. Am J Cancer Res. 2015;5:651–62.
Li J, Kurokawa M. Regulation of MDM2 stability after DNA damage. J Cell Physiol. 2015;230:2318–27.
Hinata N, Shirakawa T, Zhang Z, Matsumoto A, Fujisawa M, Okada H, et al. Radiation induces p53-dependent cell apoptosis in bladder cancer cells with wild-type- p53 but not in p53-mutated bladder cancer cells. Urol Res. 2003;31:387–96.
Kong X, Yu D, Wang Z, Li S. Relationship between p53 status and the bioeffect of ionizing radiation. Oncol Lett. 2021;22:661.
Sun Y, Ding Y, Guo C, Liu C, Ma P, Ma S, et al. α-Parvin promotes breast cancer progression and metastasis through interaction with G3BP2 and regulation of TWIST1 signaling. Oncogene. 2019;38:4856–74.
Hong HQ, Lu J, Fang XL, Zhang YH, Cai Y, Yuan J, et al. G3BP2 is involved in isoproterenol-induced cardiac hypertrophy through activating the NF-κB signaling pathway. Acta Pharm Sin. 2018;39:184–94.
Prentzell MT, Rehbein U, Cadena Sandoval M, De Meulemeester AS, Baumeister R, Brohée L, et al. G3BPs tether the TSC complex to lysosomes and suppress mTORC1 signaling. Cell. 2021;184:655–74 e627.
Kim MM, Wiederschain D, Kennedy D, Hansen E, Yuan ZM. Modulation of p53 and MDM2 activity by novel interaction with Ras-GAP binding proteins (G3BP). Oncogene. 2007;26:4209–15.
Tourrière H, Gallouzi IE, Chebli K, Capony JP, Mouaikel J, van der Geer P, et al. RasGAP-associated endoribonuclease G3Bp: selective RNA degradation and phosphorylation-dependent localization. Mol Cell Biol. 2001;21:7747–60.
Tsai WC, Reineke LC, Jain A, Jung SY, Lloyd RE. Histone arginine demethylase JMJD6 is linked to stress granule assembly through demethylation of the stress granule-nucleating protein G3BP1. J Biol Chem. 2017;292:18886–96.
Gal J, Chen J, Na DY, Tichacek L, Barnett KR, Zhu H. The acetylation of lysine-376 of G3BP1 regulates RNA binding and stress granule dynamics. Mol Cell Biol. 2019;39:e00052–19.
Angermayr M, Bandlow W. RIO1, an extraordinary novel protein kinase. FEBS Lett. 2002;524:31–6.
LaRonde-LeBlanc N, Wlodawer A. A family portrait of the RIO kinases. J Biol Chem. 2005;280:37297–300.
Angermayr M, Roidl A, Bandlow W. Yeast Rio1p is the founding member of a novel subfamily of protein serine kinases involved in the control of cell cycle progression. Mol Microbiol. 2002;44:309–24.
LaRonde-LeBlanc N, Wlodawer A. The RIO kinases: an atypical protein kinase family required for ribosome biogenesis and cell cycle progression. Biochim Biophys Acta. 2005;1754:14–24.
Line A, Slucka Z, Stengrevics A, Silina K, Li G, Rees RC. Characterisation of tumor-associated antigens in colon cancer. Cancer Immunol Immunother. 2002;51:574–82.
Weinberg F, Reischmann N, Fauth L, Taromi S, Mastroianni J, Köhler M, et al. The atypical kinase RIOK1 promotes tumor growth and invasive behavior. EBioMedicine. 2017;20:79–97.
Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, et al. Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol. 2011;29:1046–51.
Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–34.
Hong X, Huang H, Qiu X, Ding Z, Feng X, Zhu Y, et al. Targeting posttranslational modifications of RIOK1 inhibits the progression of colorectal and gastric cancers. eLife. 2018;7:e29511.
Read RD, Fenton TR, Gomez GG, Wykosky J, Vandenberg SR, Babic I, et al. A kinome-wide RNAi screen in Drosophila Glia reveals that the RIO kinases mediate cell proliferation and survival through TORC2-Akt signaling in glioblastoma. PLoS Genet. 2013;9:e1003253.
Singleton DC, Rouhi P, Zois CE, Haider S, Li JL, Kessler BM, et al. Hypoxic regulation of RIOK3 is a major mechanism for cancer cell invasion and metastasis. Oncogene. 2015;34:4713–22.
Huang Q, Lin Z, Wu P, Ni J, Shen Y. Phosphoproteomic analysis reveals Rio1-related protein phosphorylation changes in response to UV irradiation in Sulfolobus islandicus REY15A. Front Microbiol. 2020;11:586025.
Williams E, Lowe TM, Savas J, DiRuggiero J. Microarray analysis of the hyperthermophilic archaeon Pyrococcus furiosus exposed to gamma irradiation. Extremophiles. 2007;11:19–29.
Huang Q, Mayaka JB, Zhong Q, Zhang C, Hou G, Ni J, et al. Phosphorylation of the archaeal Holliday junction resolvase Hjc inhibits its catalytic activity and facilitates DNA repair in Sulfolobus islandicus REY15A. Front Microbiol. 2019;10:1214.
Vousden KH, Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–31.
Lu C, El-Deiry WS. Targeting p53 for enhanced radio- and chemo-sensitivity. Apoptosis. 2009;14:597–606.
Meek DW. Tumor suppression by p53: a role for the DNA damage response? Nat Rev Cancer. 2009;9:714–23.
Williams AB, Schumacher B. p53 in the DNA-damage-repair process. Cold Spring Harb Perspect Med. 2016;6:a026070.
Lee JT, Gu W. The multiple levels of regulation by p53 ubiquitination. Cell Death Differ. 2010;17:86–92.
Wade M, Li YC, Wahl GM. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat Rev Cancer. 2013;13:83–96.
Xie J, Li Y, Jiang K, Hu K, Zhang S, Dong X, et al. CDK16 phosphorylates and degrades p53 to promote radioresistance and predicts prognosis in lung cancer. Theranostics. 2018;8:650–62.
Kruse JP, Gu W. Modes of p53 regulation. Cell. 2009;137:609–22.
Guderian G, Peter C, Wiesner J, Sickmann A, Schulze-Osthoff K, Fischer U, et al. RioK1, a new interactor of protein arginine methyltransferase 5 (PRMT5), competes with pICln for binding and modulates PRMT5 complex composition and substrate specificity. J Biol Chem. 2011;286:1976–86.
Ferreira-Cerca S, Kiburu I, Thomson E, LaRonde N, Hurt E. Dominant Rio1 kinase/ATPase catalytic mutant induces trapping of late pre-40S biogenesis factors in 80S-like ribosomes. Nucleic Acids Res. 2014;42:8635–47.
Iacovella MG, Golfieri C, Massari LF, Busnelli S, Pagliuca C, Dal Maschio M, et al. Rio1 promotes rDNA stability and downregulates RNA polymerase I to ensure rDNA segregation. Nat Commun. 2015;6:6643.
Tourrière H, Chebli K, Zekri L, Courselaud B, Blanchard JM, Bertrand E, et al. The RasGAP-associated endoribonuclease G3BP assembles stress granules. J Cell Biol. 2003;160:823–31.
Acknowledgements
This work was supported by China Postdoctoral Science Foundation (No. 2019M663289), Natural Science Foundation of Guangdong Province (Nos. 2019A1515110144 and 2021A1515111014) and National Natural Science Foundation of China (No. 81572725).
Author information
Authors and Affiliations
Contributions
ZH and JH designed the study. YC and SZ performed most of the experiments. KW and LY performed animal experiments and constructed the expression plasmids. QO and JQ collected biological samples, analyzed the data and made the figures. CZ and HD performed Mass Spectrometry Analysis. ZH and SZ participated in writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Approvals from the ethical committee of Sun Yat-sen University Cancer Center and prior patient’s consents were previously obtained for the use of these clinical specimens for research purpose. The animal experiments were conducted according to the Animal Study Guidelines of the Ethics Committee of Sun Yat-sen University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, Y., Zhou, S., Wan, K. et al. RIOK1 mediates p53 degradation and radioresistance in colorectal cancer through phosphorylation of G3BP2. Oncogene 41, 3433–3444 (2022). https://doi.org/10.1038/s41388-022-02352-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02352-4
This article is cited by
-
High-throughput sequencing reveals Jatrorrhizine inhibits colorectal cancer growth by ferroptosis-related genes
BMC Medical Genomics (2023)
-
Hsa_circRNA_001676 accelerates the proliferation, migration and stemness in colorectal cancer through regulating miR-556-3p/G3BP2 axis
Scientific Reports (2023)
-
The RioK1 network determines p53 activity at multiple levels
Cell Death Discovery (2023)