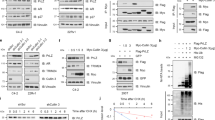
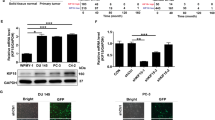
Abstract
PLK1 and Smad4 are two important factors in prostate cancer initiation and progression. They have been reported to play the opposite role in Pten-deleted mice, one is an oncogene, the other is a tumor suppressor. Moreover, they could reversely regulate the PI3K/AKT/mTOR pathway and the activation of MYC. However, the connections between PLK1 and Smad4 have never been studied. Here, we showed that PLK1 could interact with Smad4 and promote the ubiquitination and degradation of Smad4 in PCa cells. PLK1 and PELO could bind to different domains of Smad4 and formed a protein complex. PELO facilitated the degradation of Smad4 through cooperating with PLK1, thereby resulting in proliferation and metastasis of prostate cancer cell. Changes in protein levels of Smad4 led to the alteration of biological function that caused by PLK1 in prostate cancer cells. Further studies showed that PELO upregulation was positively associated with high grade PCa and knockdown of PELO expression significantly decreased PCa cell proliferation and metastasis in vitro and vivo. PELO knockdown in PCa cells could enhance the tumor suppressive role of PLK1 inhibitor. In addition, blocking the interaction between PELO and Smad4 by using specific peptide could effectively inhibit PCa cell metastasis ability in vitro and vivo. Overall, these findings identified a novel regulatory relationship among PLK1, Smad4 and PELO, and provided a potential therapeutic strategy for advanced PCa therapy by co-targeting PLK1 and PELO.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Song B, Park S-H, Zhao JC, Fong K, Li S, Lee Y, et al. Targeting FOXA1-mediated repression of TGF-β signaling suppresses castration-resistant prostate cancer progression. J Clin Investig. 2018;129:569–82.
van der Toom EE, Axelrod HD, de la Rosette JJ, de Reijke TM, Pienta KJ, Valkenburg KC. Prostate-specific markers to identify rare prostate cancer cells in liquid biopsies. Nat Rev Urol. 2019;16:7–22.
Liu XS, Song B, Elzey BD, Ratliff TL, Konieczny SF, Cheng L, et al. Polo-like Kinase 1 facilitates loss of pten tumor suppressor-induced prostate cancer formation. J Biol Chem. 2011;286:35795–35800.
Zhang Z, Hou X, Shao C, Li J, Cheng J-X, Kuang S, et al. Plk1 inhibition enhances the efficacy of androgen signaling blockade in castration-resistant prostate cancer. Cancer Res. 2014;74:6635–47.
Weichert W, Schmidt M, Gekeler V, Denkert C, Stephan C, Jung K, et al. Polo-like kinase 1 is overexpressed in prostate cancer and linked to higher tumor grades. Prostate 2004;60:240–5.
Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6:321–30.
García IA, Garro C, Fernandez E, Soria G. Therapeutic opportunities for PLK1 inhibitors: Spotlight on BRCA1-deficiency and triple negative breast cancers. Mutat Res/Fundamental Mol Mechanisms Mutagenesis. 2020;821:111693.
Ding Z, Wu C-J, Chu GC, Xiao Y. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature 2011;470:269–73.
Tan J, Li Z, Lee PL, Guan P, Aau MY, Lee ST, et al. PDK1 signaling toward PLK1–MYC activation confers oncogenic transformation, tumor-initiating cell activation, and resistance to mTOR-targeted therapy. Cancer Discov. 2013;3:1156–71.
Shi C, Yang EJ, Liu Y, Mou PK, Ren G, Shim JS. Bromodomain and extra-terminal motif (BET) inhibition is synthetic lethal with loss of SMAD4 in colorectal cancer cells via restoring the loss of MYC repression. Oncogene. 2021;40:937–50.
Adham IM, Sallam MA, Steding G, Korabiowska M, Brinck U, Hoyer-Fender S, et al. Disruption of the Pelota Gene causes early embryonic lethality and defects in cell cycle progression. Mol Cell Biol. 2003;23:1470–6.
Davis L, Engebrecht J. Yeast dom34 mutants are defective in multiple developmental pathways and exhibit decreased levels of polyribosomes. Genetics. 1998;149:45–56.
Pedersen K, Canals F, Prat A, Tabernero J, Arribas J. PELO negatively regulates HER receptor signalling and metastasis. Oncogene. 2014;33:1190–7.
Chen S, Bartkovitz D, Cai J, Chen Y, Chen Z, Chu XJ, et al. Identification of novel, potent and selective inhibitors of Polo-like kinase 1. Bioorg Med Chem Lett. 2012;22:1247–50.
Gheghiani L, Wang L, Zhang Y, Moore XTR, Zhang J, Smith SC, et al. PLK1 induces chromosomal instability and overrides cell-cycle checkpoints to drive tumorigenesis. Cancer Res. 2021;81:1293–307.
Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73–85.
Ogawa R, Yamamoto T, Hirai H, Hanada K, Kiyasu Y, Nishikawa G, et al. Loss of SMAD4 promotes colorectal cancer progression by recruiting tumor-associated neutrophils via the CXCL1/8-CXCR2 axis. Clin Cancer Res. 2019;25:2887–99.
Yao Y, Zhang Z, Kong F, Mao Z, Niu Z, Li C, et al. Smad4 induces cell death in HO-8910 and SKOV3 ovarian carcinoma cell lines via PI3K-mTOR involvement. Exp Biol Med (Maywood). 2020;245:777–84.
Zhang M, Singh R, Peng S, Mazumdar T, Sambandam V, Shen L, et al. Mutations of the LIM protein AJUBA mediate sensitivity of head and neck squamous cell carcinoma to treatment with cell-cycle inhibitors. Cancer Lett. 2017;392:71–82.
Roelen BA, Cohen OS, Raychowdhury MK, Chadee DN, Zhang Y, Kyriakis JM, et al. Phosphorylation of threonine 276 in Smad4 is involved in transforming growth factor-beta-induced nuclear accumulation. Am J Physiol Cell Physiol. 2003;285:C823–830.
Demagny H, Araki T, De Robertis EM. The tumor suppressor Smad4/DPC4 is regulated by phosphorylations that integrate FGF, Wnt, and TGF-β signaling. Cell Rep. 2014;23:688–700.
Liakath-Ali K, Mills EW, Sequeira I, Lichtenberger BM, Pisco AO, Sipilä KH, et al. An evolutionarily conserved ribosome-rescue pathway maintains epidermal homeostasis. Nature. 2018;556:376–80.
Whittaker SR, Mallinger A, Workman P, Clarke PA. Inhibitors of cyclin-dependent kinases as cancer therapeutics. Pharm Ther. 2017;173:83–105.
Drucker DJ. Advances in oral peptide therapeutics. Nat Rev Drug Discov. 2020;19:277–89.
Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov Today. 2015;20:122–8.
Olmos D, Barker D, Sharma R, Brunetto AT, Yap TA, Taegtmeyer AB, et al. Phase I study of GSK461364, a specific and competitive Polo-like kinase 1 inhibitor, in patients with advanced solid malignancies. Clin Cancer Res. 2011;17:3420–30.
García IA, Garro C, Fernandez E, Soria G. Therapeutic opportunities for PLK1 inhibitors: spotlight on BRCA1-deficiency and triple negative breast cancers. Mutat Res. 2020;821:111693.
Kumar S, Kim J. PLK-1 Targeted inhibitors and their potential against tumorigenesis. Biomed Res Int. 2015;2015:705745.
Goroshchuk O, Kolosenko I, Vidarsdottir L, Azimi A, Palm-Apergi C. Polo-like kinases and acute leukemia. Oncogene. 2019;38:1–16.
Dong X, Zhao K, Zheng W, Xu C, Zhang M, Yin R, et al. EDAG mediates Hsp70 nuclear localization in erythroblasts and rescues dyserythropoiesis in myelodysplastic syndrome. FASEB J. 2020;34:8416–27.
Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data: Figure 1. Cancer Discov. 2012;2:401–4.
Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1–pl1.
Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, et al. NCBI GEO: archive for functional genomics data sets—update. Nucleic Acids Res. 2012;41:D991–995.
Edgar R. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–10.
Acknowledgements
We thank the College of Life Sciences at Shaanxi Normal University for the sharing platform of laboratory apparatus. This work was funded by the National Natural Science Foundation of China (81972417), The “1000 Young Scholars” Program of Shaanxi Province, Natural Science Foundation of Shaanxi Province (2020JM-292, 2020JQ-430), Fundamental Research Funds for the Central Universities (GK201902002, GK201903061) and College Students’ Innovative Entrepreneurial Training Plan Program (S202110718036).
Author information
Authors and Affiliations
Contributions
XMD and PG conceived the project. PG, JLH, QWX, GQH, BBX, HH, NES, and XWD performed the experiments. QWX, HLT, and JY performed data analysis. XMD, PG, JLH, and QWX wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gao, P., Hao, JL., Xie, QW. et al. PELO facilitates PLK1-induced the ubiquitination and degradation of Smad4 and promotes the progression of prostate cancer. Oncogene 41, 2945–2957 (2022). https://doi.org/10.1038/s41388-022-02316-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02316-8
This article is cited by
-
Ubiquitination Process Mediates Prostate Cancer Development and Metastasis through Multiple Mechanisms
Cell Biochemistry and Biophysics (2024)
-
N6-methyladenosine-modified MIB1 promotes stemness properties and peritoneal metastasis of gastric cancer cells by ubiquitinating DDX3X
Gastric Cancer (2024)