Abstract
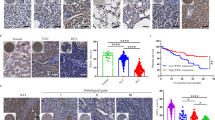
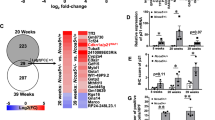
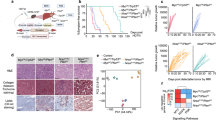
GRB2-associated-binding protein 2 (Gab2) deletion has a preventive effect of on chronic liver inflammation and hepatocellular carcinoma. This study was aimed to elaborate Gab2-initiated immunoregulation during hepatocarcinogenesis. Compared to wild-type group, liver-specific overexpression of Gab2 mice (L-Gab2) displayed early hepatocarcinogenesis after 5-month diethylnitrosamine (DEN) induction, and accelerated tumor growth after 9-month DEN challenge. More myeloid-derived suppressor cells (MDSCs) were observed in DEN-challenged L-Gab2 mice than that in DEN-treated wild-type mice. Additionally, MDSCs activation-induced tumor angiogenesis capability and immunosuppression function were exceedingly activated in DEN-exposed L-Gab2 mice, which reflected in the increased platelet endothelial cell adhesion molecule (PECAM) and vascular endothelial growth factor (VEGF), and the decreased cytotoxic T lymphocytes. Mechanistically, DEN-challenged L-Gab2 mice produced more IL-6, and IL-6 depletion significantly deprived Gab2-overexpression-mediated tumor-promotion phenomena, accompanied by the impairment of MDSCs-initiated immunosuppression function. MDSCs isolated from IL-6-depleted L-Gab2 mice or inactivating MDSCs partly restored the immune function of cytotoxic T cells. Of note, MDSCs gene signatures had a significant association with the increased Gab2 or IL6 in hepatoma specimens. Collectively, L-Gab2 mice accelerated hepatoma progression possibly through activating IL-6-initiated the activation of MDSCs. This study provides a novel insights for exploring the role of Gab2 in autoimmune tolerance during hepatocarcinogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data were generated by the authors and included in the article.
Change history
06 May 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41388-022-02337-3
References
Chen Z, Xie H, Hu M, Huang T, Hu Y, Sang N, et al. Recent progress in treatment of hepatocellular carcinoma. Am J Cancer Res. 2020;10:2993–3036.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics, 2012. Ca-Cancer J Clin. 2015;65:87–108.
Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology 2013;144:512–27.
Saviano A, Roehlen N, Virzi A, Roca Suarez AA, Hoshida Y, Lupberger J, et al. Stromal and Immune Drivers of Hepatocarcinogenesis. In: Hoshida Y, editor. Hepatocellular Carcinoma: Translational Precision Medicine Approaches. Cham (CH)2019. p. 317–31.
Triantafyllou E, Woollard KJ, McPhail MJW, Antoniades CG, Possamai LA. The role of monocytes and macrophages in acute and acute-on-chronic liver failure. Front Immunol. 2018;9:2948.
Schrader J. The role of MDSCs in hepatocellular carcinoma-in vivo veritas? J Hepatol. 2013;59:921–3.
Waldron TJ, Quatromoni JG, Karakasheva TA, Singhal S, Rustgi AK. Myeloid derived suppressor cells: Targets for therapy. Oncoimmunology 2013;2:e24117.
Atretkhany KN, Drutskaya MS. Myeloid-derived suppressor cells and proinflammatory cytokines as targets for cancer therapy. Biochem (Mosc). 2016;81:1274–83.
Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25.
Beury DW, Parker KH, Nyandjo M, Sinha P, Carter KA, Ostrand-Rosenberg S. Cross-talk among myeloid-derived suppressor cells, macrophages, and tumor cells impacts the inflammatory milieu of solid tumors. J Leukoc Biol. 2014;96:1109–18.
Ke Y, Wu D, Princen F, Nguyen T, Pang Y, Lesperance J, et al. Role of Gab2 in mammary tumorigenesis and metastasis. Oncogene 2007;26:4951–60.
Ding CB, Luo JM, Yu WN, Gao SY, Yang LW, Chen C, et al. Gab2 is a novel prognostic factor for colorectal cancer patients. Int J Clin Exp Patho. 2015;8:2779–86.
Lee AW, Mao Y, Penninger JM, Yu S. Gab2 promotes colony-stimulating factor 1-regulated macrophage expansion via alternate effectors at different stages of development. Mol Cell Biol. 2011;31:4563–81.
Guo X, Li T, Xu Y, Xu X, Zhu Z, Zhang Y, et al. Increased levels of Gab1 and Gab2 adaptor proteins skew interleukin-4 (IL-4) signaling toward M2 macrophage-driven pulmonary fibrosis in mice. J Biol Chem. 2017;292:14003–15.
Ding CB, Yu WN, Feng JH, Luo JM. Structure and function of Gab2 and its role in cancer (Review). Mol Med Rep. 2015;12:4007–14.
Cheng J, Zhong Y, Chen S, Sun Y, Huang L, Kang Y, et al. Gab2 mediates hepatocellular carcinogenesis by integrating multiple signaling pathways. Faseb J. 2017;31:5530–42.
Zanconato F, Cordenonsi M, Piccolo S. YAP and TAZ: A signalling hub of the tumour microenvironment. Nat Rev Cancer. 2019;19:454–64.
Choo YW, Kang M, Kim HY, Han J, Kang S, Lee JR, et al. M1 macrophage-derived nanovesicles potentiate the anticancer efficacy of immune checkpoint inhibitors. ACS Nano. 2018;12:8977–93.
Chang WL, Masih S, Thadi A, Patwa V, Joshi A, Cooper HS, et al. Plecanatide-mediated activation of guanylate cyclase-C suppresses inflammation-induced colorectal carcinogenesis in Apc(+/Min-FCCC) mice. World J Gastrointest Pharm Ther. 2017;8:47–59.
Zhang YS, Wang F, Cui SX, Qu XJ. Natural dietary compound naringin prevents azoxymethane/dextran sodium sulfate-induced chronic colorectal inflammation and carcinogenesis in mice. Cancer Biol Ther. 2018;19:735–44.
Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008;135:234–43.
Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, et al. Increase in CD14(+)HLA-DR-/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immun. 2013;62:1421–30.
Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009;50:799–807.
Bocanegra M, Bergamaschi A, Kim YH, Miller MA, Rajput AB, Kao J, et al. Focal amplification and oncogene dependency of GAB2 in breast cancer. Oncogene 2010;29:774–9.
Zatkova A, Schoch C, Speleman F, Poppe B, Mannhalter C, Fonatsch C, et al. GAB2 is a novel target of 11q amplification in AML/MDS. Gene Chromosome Canc. 2006;45:798–807.
Chernoff KA, Bordone L, Horst B, Simon K, Twadell W, Lee K, et al. GAB2 amplifications refine molecular classification of melanoma. Clin Cancer Res. 2009;15:4288–91.
Horst B, Gruvberger-Saal SK, Hopkins BD, Bordone L, Yang Y, Chernoff KA, et al. Gab2-mediated signaling promotes melanoma metastasis. Am J Pathol. 2009;174:1524–33.
Yang Y, Wu J, Demir A, Castillo-Martin M, Melamed RD, Zhang G, et al. GAB2 induces tumor angiogenesis in NRAS-driven melanoma. Oncogene 2013;32:3627–37.
Park YR, Bae SH, Ji W, Seo EJ, Lee JC, Kim HR, et al. GAB2 amplification in squamous cell lung cancer of non-smokers. J Korean Med Sci. 2017;32:1784–91.
Herr R, Halbach S, Heizmann M, Busch H, Boerries M, Brummer T. BRAF inhibition upregulates a variety of receptor tyrosine kinases and their downstream effector Gab2 in colorectal cancer cell lines. Oncogene. 2018;37:1576-93
Kapanadze T, Gamrekelashvili J, Ma C, Chan C, Zhao F, Hewitt S, et al. Regulation of accumulation and function of myeloid derived suppressor cells in different murine models of hepatocellular carcinoma. J Hepatol. 2013;59:1007–13.
Zhao F, Hoechst B, Duffy A, Gamrekelashvili J, Fioravanti S, Manns MP, et al. S100A9 a new marker for monocytic human myeloid-derived suppressor cells. Immunology 2012;136:176–83.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74.
Ribechini E, Greifenberg V, Sandwick S, Lutz M. Subsets, expansion and activation of myeloid-derived suppressor cells. Med Microbiol Immun. 2010;199:273–81.
Brodaczewska KK, Donskow-Lysoniewska K, Krawczak K, Doligalska M. Role of l-arginine and CD11b+Gr-1+ cells in immunosuppression induced by Heligmosomoides polygyrus bakeri. Parasite Immunol. 2020;42:e12704.
Lu LC, Chang CJ, Hsu CH. Targeting myeloid-derived suppressor cells in the treatment of hepatocellular carcinoma: Current state and future perspectives. J Hepatocell Carcinoma. 2019;6:71–84.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74.
Deng Y, Cheng J, Fu B, Liu W, Chen G, Zhang Q, et al. Hepatic carcinoma-associated fibroblasts enhance immune suppression by facilitating the generation of myeloid-derived suppressor cells. Oncogene 2017;36:1090–101.
Chen S, Kang Y, Sun Y, Zhong Y, Li Y, Deng L, et al. Deletion of Gab2 in mice protects against hepatic steatosis and steatohepatitis: A novel therapeutic target for fatty liver disease. J Mol Cell Biol. 2016;8:492–504.
De Cicco P, Ercolano G, Ianaro A. The new era of cancer immunotherapy: Targeting myeloid-derived suppressor cells to overcome immune evasion. Front Immunol. 2020;11:1680.
Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, et al. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120:16–25.
Urakawa S, Yamasaki M, Goto K, Haruna M, Hirata M, Morimoto-Okazawa A, et al. Peri-operative monocyte count is a marker of poor prognosis in gastric cancer: increased monocytes are a characteristic of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2019;68:1341–50.
Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–D7.
Acknowledgements
We thank the Gene Expression Profiling Interactive Analysis 2.0 online database (GEPIA2). This work was supported by the grants from Natural Science Foundation of China (81802332 and 82103189), Natural Science Foundation of Fujian Province (2020J05302 and 2021J011358), Science Foundation of the Fujian provincial Commission of Health and Family Planning (2021GGB026) and Natural Science Basic Research Program of Shaanxi Province (2021JQ-780).
Author information
Authors and Affiliations
Contributions
ZL and XL designed the study; SC and JC performed research and drafted the paper; YZ and RL participated in the exploration of MDSCs function and helped in histology and IHC staining; SC and JC performed clinical correlation analysis; SC and RL contributed to the statistical analysis, and all authors polished and approved the final paper.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
We declare that the study includes a statement on ethics approval and consent.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, S., Cheng, J., Zhong, Y. et al. Liver-specific overexpression of Gab2 accelerates hepatocellular carcinoma progression by activating immunosuppression of myeloid-derived suppressor cells. Oncogene 41, 3316–3327 (2022). https://doi.org/10.1038/s41388-022-02298-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02298-7