Abstract
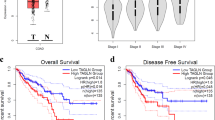
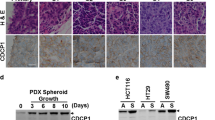
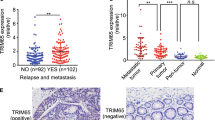
Trop-2 is a transmembrane signal transducer that is overexpressed in most human cancers, and drives malignant progression. To gain knowledge on the higher-order molecular mechanisms that drive Trop-2 signaling, we applied next-generation sequencing, proteomics, and high-resolution microscopy to models and primary cases of human colorectal cancer (CRC). We had previously shown that Trop-2 induces a Ca2+ signal. We reveal here that Trop-2 binds the cell membrane Na+/K+-ATPase, and that clustering of Trop-2 induces an intracellular Ca2+ rise followed by membrane translocation of PKCα, which in turn phosphorylates the Trop-2 cytoplasmic tail. This feed-forward signaling is promoted by the binding of Trop-2 to the PKCα membrane-anchor CD9. CRISPR-based inactivation of CD9 in CRC cells shows that CD9 is required by Trop-2 for recruiting PKCα and cofilin-1 to the cell membrane. This induces malignant progression through proteolytic cleavage of E-cadherin, remodeling of the β-actin cytoskeleton, and activation of Akt and ERK. The interaction between Trop-2 and CD9 was validated in vivo in murine models of CRC growth and invasion. Overexpression of the components of this Trop-2-driven super-complex significantly worsened disease-free and overall survival of CRC patients, supporting a pivotal relevance in CRC malignant progression. Our findings demonstrate a previously unsuspected layer of cancer growth regulation, which is dormant in normal tissues, and is activated by Trop-2 in cancer cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Guerra E, Trerotola M, Aloisi AL, Tripaldi R, Vacca G, La Sorda R, et al. The Trop-2 signalling network in cancer growth. Oncogene. 2013;32:1594–600.
Trerotola M, Cantanelli P, Guerra E, Tripaldi R, Aloisi AL, Bonasera V, et al. Up-regulation of Trop-2 quantitatively stimulates human cancer growth. Oncogene. 2013;32:222–33.
Guerra E, Trerotola M, Tripaldi R, Aloisi AL, Simeone P, Sacchetti A, et al. Trop-2 induces tumor growth through Akt and determines sensitivity to Akt inhibitors. Clin Cancer Res. 2016;22:4197–205.
Trerotola M, Li J, Alberti S, Languino LR. Trop-2 inhibits prostate cancer cell adhesion to fibronectin through the β1 integrin-RACK1 axis. J Cell Physiol. 2012;227:3670–7.
Guerra E, Trerotola M, Dell’ Arciprete R, Bonasera V, Palombo B, El-Sewedy T, et al. A bi-cistronic CYCLIN D1-TROP2 mRNA chimera demonstrates a novel oncogenic mechanism in human cancer. Cancer Res. 2008;68:8113–21.
Trerotola M, Guerra E, Ali Z, Aloisi AL, Ceci M, Simeone P, et al. Trop-2 cleavage by ADAM10 is an activator switch for cancer growth and metastasis. Neoplasia. 2021;23:415–28.
Rios-Doria J, Day KC, Kuefer R, Rashid MG, Chinnaiyan AM, Rubin MA, et al. The role of calpain in the proteolytic cleavage of E-cadherin in prostate and mammary epithelial cells. J Biol Chem. 2003;278:1372–9.
Maretzky T, Reiss K, Ludwig A, Buchholz J, Scholz F, Proksch E, et al. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and beta-catenin translocation. Proc Natl Acad Sci USA. 2005;102:9182–7.
Guerra E, Trerotola M, Relli V, Lattanzio R, Tripaldi R, Vacca G, et al. Trop-2 induces ADAM10-mediated cleavage of E-cadherin and drives EMT-less metastasis in colon cancer. Neoplasia. 2021;23:898–911.
Ripani E, Sacchetti A, Corda D, Alberti S. The human Trop-2 is a tumor-associated calcium signal transducer. Int J Cancer. 1998;76:671–6.
Thomas A, Pommier Y. Targeting topoisomerase I in the era of precision medicine. Clin Cancer Res. 2019;25:6581–9.
Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380:741–51.
Fornaro M, Dell’Arciprete R, Stella M, Bucci C, Nutini M, Capri MG, et al. Cloning of the gene encoding TROP-2, a cell-surface glycoprotein expressed by human carcinomas. Int J Cancer. 1995;62:610–8.
El Sewedy T, Fornaro M, Alberti S. Cloning of the murine Trop2 gene: conservation of a PIP2-binding sequence in the cytoplasmic domain of Trop-2. Int J Cancer. 1998;75:324–30.
Alberti S, Miotti S, Stella M, Klein CE, Fornaro M, Ménard S, et al. Biochemical characterization of Trop-2, a cell surface molecule expressed by human carcinomas: formal proof that the monoclonal antibodies T16 and MOv-16 recognize Trop-2. Hybridoma. 1992;11:539–5.
Moore EDW, Etter EF, Philipson KD, Carrington WA, Fogarty KE, Lifshitz LM, et al. Coupling of the Na+/Ca2+exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature. 1993;365:657–60.
Liang M, Tian J, Liu L, Pierre S, Liu J, Shapiro J, et al. Identification of a Pool of Non-pumping Na/K-ATPase. J Biol Chem. 2007;282:10585–93.
Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, et al. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell. 2005;17:317–26.
Tian J, Xie ZJ. The Na-K-ATPase and calcium-signaling microdomains. Physiology. 2008;23:205–11.
Matchkov VV, Krivoi II. Specialized functional diversity and interactions of the Na,K-ATPase. Front Physiol. 2016;7:179.
Haas M, Wang H, Tian J, Xie Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem. 2002;277:18694–702.
Shen J-J, Zhan Y-C, Li H-Y, Wang Z. Ouabain impairs cancer metabolism and activates AMPK-Src signaling pathway in human cancer cell lines. Acta Pharmacol Sin. 2020;41:110–8.
Chen D, Song M, Mohamad O, Yu SP. Inhibition of Na+/K+-ATPase induces hybrid cell death and enhanced sensitivity to chemotherapy in human glioblastoma cells. BMC Cancer. 2014;14:716.
Tian J, Li X, Liang M, Liu L, Xie JX, Ye Q, et al. Changes in sodium pump expression dictate the effects of ouabain on cell growth. J Biol Chem. 2009;284:14921–9.
Ninsontia C, Chanvorachote P. Ouabain mediates integrin switch in human lung cancer cells. Anticancer Res. 2014;34:5495.
Larre I, Lazaro A, Contreras RG, Balda MS, Matter K, Flores-Maldonado C, et al. Ouabain modulates epithelial cell tight junction. Proc Natl Acad Sci. 2010;107:11387.
Morikawa K, Walker SM, Nakajima M, Pathak S, Jessup JM, Fidler IJ. Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 1988;48:6863–71.
Basu A, Goldenberg DM, Stein R. The epithelial/carcinoma antigen EGP-1, recognized by monoclonal antibody RS7-3G11, is phosphorylated on serine 303. Int J Cancer. 1995;62:472–9.
Wanger TM, Dewitt S, Collins A, Maitland NJ, Poghosyan Z, Knauper V. Differential regulation of TROP2 release by PKC isoforms through vesicles and ADAM17. Cell Signal. 2015;27:1325–35.
Mori Y, Akita K, Ojima K, Iwamoto S, Yamashita T, Morii E, et al. Trophoblast cell surface antigen 2 (Trop-2) phosphorylation by protein kinase C alpha/delta (PKCalpha/delta) enhances cell motility. J Biol Chem. 2019;294:11513–24.
Farah CA, Sossin WS. The role of C2 domains in PKC signaling. Adv Exp Med Biol. 2012;740:663–83.
Alberti S. A phosphoinositide-binding sequence is shared by PH domain target molecules–a model for the binding of PH domains to proteins. Proteins. 1998;31:1–9.
Alberti S. HIKE, a candidate protein binding site for PH domains, is a major regulatory region of Gbeta proteins. Proteins. 1999;35:360–3.
Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169:381–405.
Freeley M, Kelleher D, Long A. Regulation of protein kinase C function by phosphorylation on conserved and non-conserved sites. Cell Signal. 2011;23:753–62.
Tanaka K, Babic I, Nathanson D, Akhavan D, Guo D, Gini B, et al. Oncogenic EGFR signaling activates an mTORC2-NF-κB pathway that promotes chemotherapy resistance. Cancer Discov. 2011. https://doi.org/10.1158/2159-8290.CD-11-0124.
Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302.
Trerotola M, Ganguly KK, Fazli L, Fedele C, Lu H, Dutta A, et al. Trop-2 is up-regulated in invasive prostate cancer and displaces FAK from focal contacts. Oncotarget. 2015.
Trerotola M, Jernigan D, Liu Q, Siddiqui J, Fatatis A, Languino L. Trop-2 promotes prostate cancer metastasis by modulating β1 integrin functions. Cancer Res. 2013;73:3155–67.
van Rheenen J, Song X, van Roosmalen W, Cammer M, Chen X, DesMarais V, et al. EGF-induced PIP2 hydrolysis releases and activates cofilin locally in carcinoma cells. J Cell Biol. 2007;179:1247–59.
Spratley SJ, Bastea LI, Doppler H, Mizuno K, Storz P. Protein kinase D regulates cofilin activity through p21-activated kinase 4. J Biol Chem. 2011;286:34254–61.
Sakuma M, Shirai Y, Yoshino K-I, Kuramasu M, Nakamura T, Yanagita T, et al. Novel PKCα-mediated phosphorylation site(s) on cofilin and their potential role in terminating histamine release. Mol Biol Cell. 2012;23:3707–21.
Saczko-Brack D, Warchol E, Rogez B, Kross M, Heissler SM, Sellers JR, et al. Self-organization of actin networks by a monomeric myosin. Proc Natl Acad Sci USA. 2016;113:E8387–95.
Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and α-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008;10:1039–50.
Watanabe T, Hosoya H, Yonemura S. Regulation of myosin II dynamics by phosphorylation and dephosphorylation of its light chain in epithelial cells. Mol Biol Cell. 2007;18:605–16.
Zanna P, Trerotola M, Vacca G, Bonasera V, Palombo B, Guerra E, et al. Trop-1 are conserved growth stimulatory molecules that mark early stages of tumor progression. Cancer. 2007;110:452–64.
Ng T, Shima D, Squire A, Bastiaens PIH, Gschmeissner S, Humphries MJ, et al. PKCα regulates β1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 1999;18:3909–23.
Zhang XA, Bontrager AL, Hemler ME. Transmembrane-4 superfamily proteins associate with activated protein kinase C (PKC) and link PKC to specific beta(1) integrins. J Biol Chem. 2001;276:25005–13.
Jiang X, Teng M, Ji R, Zhang D, Zhang Z, Lv Y, et al. CD9 regulates keratinocyte differentiation and motility by recruiting E-cadherin to the plasma membrane and activating the PI3K/Akt pathway. Biochim Biophys Acta. 2020;1867:118574.
Cabantous S, Nguyen HB, Pedelacq J-D, Koraïchi F, Chaudhary A, Ganguly K, et al. A new protein-protein interaction sensor based on tripartite split-GFP association. Sci Rep. 2013;3:2854.
Wang H-X, Kolesnikova TV, Denison C, Gygi SP, Hemler ME. The C-terminal tail of tetraspanin protein CD9 contributes to its function and molecular organization. J Cell Sci. 2011;124:2702–10.
Sveen A, Ågesen TH, Nesbakken A, Rognum TO, Lothe RA, Skotheim RI. Transcriptome instability in colorectal cancer identified by exon microarray analyses: associations with splicing factor expression levels and patient survival. Genome Med. 2011;3:32.
Ågesen TH, Sveen A, Merok MA, Lind GE, Nesbakken A, Skotheim RI, et al. ColoGuideEx: a robust gene classifier specific for stage II colorectal cancer prognosis. Gut. 2012;61:1560.
Ambrogi F, Fornili M, Boracchi P, Trerotola M, Relli V, Simeone P, et al. Trop-2 is a determinant of breast cancer survival. PLoS ONE. 2014;9:e96993.
Stoyanova T, Goldstein AS, Cai H, Drake JM, Huang J, Witte ON. Regulated proteolysis of Trop2 drives epithelial hyperplasia and stem cell self-renewal via beta-catenin signaling. Genes Dev. 2012;26:2271–85.
Mochly-Rosen D, Gordon AS. Anchoring proteins for protein kinase C: a means for isozyme selectivity. FASEB J. 1998;12:35–42.
Murayama Y, Shinomura Y, Oritani K, Miyagawa J, Yoshida H, Nishida M, et al. The tetraspanin CD9 modulates epidermal growth factor receptor signaling in cancer cells. J Cell Physiol. 2008;216:135–43.
Powner D, Kopp Petra M, Monkley Susan J, Critchley David R, Berditchevski F. Tetraspanin CD9 in cell migration. Biochem Soc Trans. 2011;39:563–7.
Hoshino A, Kim HS, Bojmar L, Gyan KE, Cioffi M, Hernandez J, et al. Extracellular vesicle and particle biomarkers define multiple human cancers. Cell. 2020;182:1044–61.e18.
Krishn SR, Singh A, Bowler N, Duffy AN, Friedman A, Fedele C, et al. Prostate cancer sheds the αvβ3 integrin in vivo through exosomes. Matrix Biol. 2019;77:41–57.
Ciccarelli F, Acciarito A, Alberti S. Large and diverse numbers of human diseases with HIKE mutations. Hum Mol Genet. 2000;9:1001–7.
Antal CE, Hudson AM, Kang E, Zanca C, Wirth C, Stephenson NL, et al. Cancer-associated protein kinase C mutations reveal kinase’s role as tumor suppressor. Cell. 2015;160:489–502.
Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–4.
Bahmad HF, Cheaito K, Chalhoub RM, Hadadeh O, Monzer A, Ballout F, et al. Sphere-formation assay: three-dimensional in vitro culturing of prostate cancer stem/progenitor sphere-forming cells. Front Oncol. 2018;8:347.
Scott RW, Hooper S, Crighton D, Li A, Konig I, Munro J, et al. LIM kinases are required for invasive path generation by tumor and tumor-associated stromal cells. J Cell Biol. 2010;191:169–85.
Di Baldassarre A, D’Amico MA, Izzicupo P, Gaggi G, Guarnieri S, Mariggiò MA, et al. Cardiomyocytes derived from human cardiopoieticamniotic fluids. Sci Rep. 2018;8:12028.
Acknowledgements
We thank G. Vacca, F. Dini, E. Eleuterio, and S. Angelucci for help during the course of this work. We also thank C. Berrie for language editing of the paper.
Funding
Italian Ministry of Health (RicOncol RF-EMR-2006-361866), Italian Ministry of Development—FESR 2016–2018. SSI000651, art. 69 Reg. (CE) n. 1083/2006 and Reg. (CE) n. 1828/2006, Region Abruzzo (POR FESR 2007–2013: Activity 1.1.1 line B) C78C14000100005, Oncoxx Biotech, Italy and Marie Curie Transfer of Knowledge Fellowship—EC VI Framework Program (Contract 014541) to SA. Programma Per Giovani Ricercatori “Rita Levi Montalcini”, Italian Ministry of University and Research (Grant PGR12I7N1Z) to MT. EG is an inventor in patents WO201687651 and WO201784763, and a partner in Mediterranea Theranostic Srl. MT is an inventor in patent WO201784763. SA is an inventor in patents WO201089782, WO201687651, and WO201784763, and is founder and CEO of Oncoxx Biotech Srl and Mediterranea Theranostic Srl. VR was an employee of Oncoxx Biotech Srl. PS is an inventor in a patent application under consideration (EP3546948).
Author information
Authors and Affiliations
Contributions
MT, VR, RT, AS, KH, PS, EG, MC, and and ALA performed the biochemical and functional assays; RLS, RL, NT, and MP collected the human tumor samples, assembled tissue microarrays, and performed IHC analysis; DV. performed the 2D-PAGE and the NanoLC-MS/MS assays; DV, IF, and MS analyzed the data from NanoLC and MS/MS assays; MT and SA planned the study and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All procedures involving animal care were approved by the Interuniversity Animal Research Ethics Committee (CEISA), Chieti-Pescara and Teramo Universities (Prot. 26/2011/CEISA/PROG/16), and were conducted in compliance with consensus international protocols (D.L. No. 116, G.U. Feb. 18,1992; No. 8, G.U. July, 1994; UKCCCR Guidelines for the Welfare of Animals in Experimental Neoplasia; EEC Council Directive 86/609, Dec. 12,1987; Guide for the Care and Use of Laboratory Animals, US-NRC,1996). Studies on human tumor samples were approved by the Italian Ministry of Health (RicOncol RF-EMR-2006-361866).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guerra, E., Relli, V., Ceci, M. et al. Trop-2, Na+/K+ ATPase, CD9, PKCα, cofilin assemble a membrane signaling super-complex that drives colorectal cancer growth and invasion. Oncogene 41, 1795–1808 (2022). https://doi.org/10.1038/s41388-022-02220-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-022-02220-1
This article is cited by
-
ATF2 loss promotes tumor invasion in colorectal cancer cells via upregulation of cancer driver TROP2
Cellular and Molecular Life Sciences (2022)