Abstract
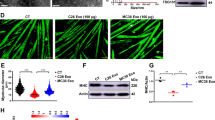
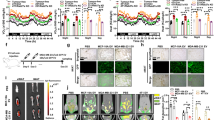
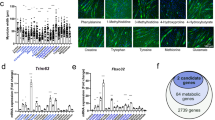
Tumor-derived exosomes are emerging mediators of cancer cachexia. Clarifying the regulation of exosome biogenesis and finding possible targets for cancer cachexia therapy are important and necessary. In the present study, systemic analysis of the roles of STAT3 in controlling exosome biogenesis of murine C26 colon tumor cells and its contribution to the development of cancer cachexia is conducted. The genetic manipulation of STAT3 expression, STAT3 knockout (KO) or overexpression (OE), significantly affected the exosome biogenesis and also the potency of C26 conditioned medium (CM) in inducing muscle atrophy and lipolysis in vitro. The genetic manipulation of STAT3 expression caused change in phosphorylation of PKM2 and glycolysis. PKM2/SNAP23 pathway was involved in regulation of exosome biogenesis by STAT3 genetic manipulation as well as by STAT3 inhibitors in C26 cells. Mice inoculated with STAT3 knockout or overexpression C26 cells exhibited ameliorated or aggravated cancer cachexia symptoms, with a positive correlation with the serum exosome and IL-6 levels. The STAT3/PKM2/SNAP23 pathway was affected in C26 tumor tissues with genetic manipulation of STAT3 expression. The capacity of exosome biogenesis of different human cancer cells also exhibited a positive correlation with the activation of STAT3/PKM2/SNAP23 pathway. The research presented here confirms that STAT3 plays a critical role in regulating biogenesis of tumor-derived exosomes which could contribute to cancer cachexia development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Baracos VE, Martin L, Korc M, Guttridge DC, Fearon KCH. Cancer-associated cachexia. Nat Rev Dis Prim. 2018;4:17105.
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–95.
Prado BL, Qian Y. Anti-cytokines in the treatment of cancer cachexia. Ann Palliat Med. 2019;8:67–79.
Gao X, Wang Y, Lu F, Chen X, Yang D, Cao Y, et al. Extracellular vesicles derived from oesophageal cancer containing P4HB promote muscle wasting via regulating PHGDH/Bcl-2/caspase-3 pathway. J Extracell Vesicles. 2021;10:e12060.
Chitti SV, Fonseka P, Mathivanan S. Emerging role of extracellular vesicles in mediating cancer cachexia. Biochem Soc Trans. 2018;46:1129–36.
Marinho R, Alcântara PSM, Ottoch JP, Seelaender M. Role of Exosomal MicroRNAs and myomiRs in the development of cancer cachexia-associated muscle wasting. Front Nutr. 2017;4:69.
Hu W, Ru Z, Xiao W, Xiong Z, Wang C, Yuan C, et al. Adipose tissue browning in cancer-associated cachexia can be attenuated by inhibition of exosome generation. Biochem Biophys Res Commun. 2018;506:122–9.
Zhou L, Zhang T, Shao W, Lu R, Wang L, Liu H, et al. Amiloride ameliorates muscle wasting in cancer cachexia through inhibiting tumor-derived exosome release. Skelet Muscle. 2021;11:17.
Yáñez-Mó M, Siljander PR, Andreu Z, Zavec AB, Borràs FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066.
Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–79.
Simon T, Jackson E, Giamas G. Breaking through the glioblastoma micro-environment via extracellular vesicles. Oncogene. 2020;39:4477–90.
Wendler F, Favicchio R, Simon T, Alifrangis C, Stebbing J, Giamas G. Extracellular vesicles swarm the cancer microenvironment: from tumor-stroma communication to drug intervention. Oncogene. 2017;36:877–84.
D’Souza-Schorey C, Clancy JW. Tumor-derived microvesicles: shedding light on novel microenvironment modulators and prospective cancer biomarkers. Genes Dev. 2012;26:1287–99.
Kunou S, Shimada K, Takai M, Sakamoto A, Aoki T, Hikita T, et al. Exosomes secreted from cancer-associated fibroblasts elicit anti-pyrimidine drug resistance through modulation of its transporter in malignant lymphoma. Oncogene. 2021;40:3989–4003.
Yang X, Chen Y, Zhou Y, Wu C, Li Q, Wu J, et al. GPC5 suppresses lung cancer progression and metastasis via intracellular CTDSP1/AhR/ARNT signaling axis and extracellular exosome secretion. Oncogene. 2021;40:4307–23.
Miao C, Zhang W, Feng L, Gu X, Shen Q, Lu S, et al. Cancer-derived exosome miRNAs induce skeletal muscle wasting by Bcl-2-mediated apoptosis in colon cancer cachexia. Mol Ther - Nucleic Acids. 2021;24:923–38.
Harada Y, Nakajima K, Suzuki T, Fukushige T, Kondo K, Seino J, et al. Glycometabolic regulation of the biogenesis of small extracellular vesicles. Cell Rep. 2020;33:108261.
Rice GE, Scholz-Romero K, Sweeney E, Peiris H, Kobayashi M, Duncombe G, et al. The effect of glucose on the release and bioactivity of exosomes from first trimester trophoblast cells. J Clin Endocrinol Metab. 2015;100:E1280–88.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977.
McAndrews KM, Kalluri R. Mechanisms associated with biogenesis of exosomes in cancer. Mol Cancer. 2019;18:52.
Wei Y, Wang D, Jin F, Bian Z, Li L, Liang H, et al. Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nat Commun. 2017;8:14041.
Chai EZ, Shanmugam MK, Arfuso F, Dharmarajan A, Wang C, Kumar AP, et al. Targeting transcription factor STAT3 for cancer prevention and therapy. Pharm Ther. 2016;162:86–97.
Valle-Mendiola A, Soto-Cruz I. Energy metabolism in cancer: the roles of STAT3 and STAT5 in the regulation of metabolism-related genes. Cancers. 2020;12:124.
Miller A, McLeod L, Alhayyani S, Szczepny A, Watkins DN, Chen W, et al. Blockade of the IL-6 trans-signalling/STAT3 axis suppresses cachexia in Kras-induced lung adenocarcinoma. Oncogene. 2017;36:3059–66.
Zimmers TA, Fishel ML, Bonetto A. STAT3 in the systemic inflammation of cancer cachexia. Semin Cell Dev Biol. 2016;54:28–41.
Eskiler GG, Bezdegumeli E, Ozman Z, Ozkan AD, Bilir C, Kucukakca BN, et al. IL-6 mediated JAK/STAT3 signaling pathway in cancer patients with cachexia. Bratisl Lek Listy. 2019;66:819–26.
Seto DN, Kandarian SC, Jackman RW. A key role for leukemia inhibitory factor in C26 cancer cachexia. J Biol Chem. 2015;290:19976–86.
Jaśkiewicz A, Domoradzki T, Pająk B. Targeting the JAK2/STAT3 pathway-can we compare it to the two faces of the God Janus? Int J Mol Sci.2020;21:8261.
Arora G, Gupta A, Guo T, Gandhi A, Laine A, Williams D, et al. JAK inhibitors suppress cancer cachexia-associated anorexia and adipose wasting in mice. JCSM Rapid Commun. 2020;3:115–28.
Chen L, Yang Q, Zhang H, Wan L, Xin B, Cao Y, et al. Cryptotanshinone prevents muscle wasting in CT26-induced cancer cachexia through inhibiting STAT3 signaling pathway. J Ethnopharmacol. 2020;260:113066.
Chen L, Xu W, Yang Q, Zhang H, Wan L, Xin B, et al. Imperatorin alleviates cancer cachexia and prevents muscle wasting via directly inhibiting STAT3. Pharm Res. 2020;158:104871.
Shouda T, Hiraoka K, Komiya S, Hamada T, Zenmyo M, Iwasaki H, et al. Suppression of IL-6 production and proliferation by blocking STAT3 activation in malignant soft tissue tumor cells. Cancer Lett. 2006;231:176–84.
Shin MK, Sasaki F, Ki DW, Win NN, Morita H, Hayakawa Y. Identification of Ophiocordyceps gracilioides by its anti-tumor effects through targeting the NFκB-STAT3-IL-6 inflammatory pathway. Biol Pharm Bull. 2021;44:686–90.
Rodriguez-Barrueco R, Yu J, Saucedo-Cuevas LP, Olivan M, Llobet-Navas D, Putcha P, et al. Inhibition of the autocrine IL-6-JAK2-STAT3-calprotectin axis as targeted therapy for HR-/HER2+ breast cancers. Genes Dev. 2015;29:1631–48.
Kroon P, Berry PA, Stower MJ, Rodrigues G, Mann VM, Simms M, et al. JAK-STAT blockade inhibits tumor initiation and clonogenic recovery of prostate cancer stem-like cells. Cancer Res. 2013;73:5288–98.
Dorayappan KDP, Wanner R, Wallbillich JJ, Saini U, Zingarelli R, Suarez AA, et al. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: a novel mechanism linking STAT3/Rab proteins. Oncogene. 2018;37:3806–21.
Ren R, Sun H, Ma C, Liu J, Wang H. Colon cancer cells secrete exosomes to promote self-proliferation by shortening mitosis duration and activation of STAT3 in a hypoxic environment. Cell Biosci. 2019;9:62.
Kosgodage US, Mould R, Henley AB, Nunn AV, Guy GW, Thomas EL, et al. Cannabidiol (CBD) is a novel inhibitor for Exosome and Microvesicle (EMV) release in cancer. Front Pharm. 2018;9:889.
Silva KA, Dong J, Dong Y, Dong Y, Schor N, Tweardy DJ, et al. Inhibition of Stat3 activation suppresses caspase-3 and the ubiquitin-proteasome system, leading to preservation of muscle mass in cancer cachexia. J Biol Chem. 2015;290:11177–87.
Zhang WL, Li N, Shen Q, Fan M, Guo XD, Zhang XW, et al. Establishment of a mouse model of cancer cachexia with spleen deficiency syndrome and the effects of atractylenolide I. Acta Pharm Sin. 2020;41:237–48.
Fu XQ, Chou JY, Li T, Zhu PL, Li JK, Yin CL, et al. The JAK2/STAT3 pathway is involved in the anti-melanoma effects of atractylenolide I. Exp Dermatol. 2018;27:201–4.
Liu Y, Jia Z, Dong L, Wang R, Qiu G. A randomized pilot study of atractylenolide I on gastric cancer cachexia patients. Evid Based Complement Altern Med. 2008;5:337–44.
Suzuki T, Von Haehling S, Springer J. Promising models for cancer-induced cachexia drug discovery. Expert Opin Drug Disco. 2020;15:627–37.
Kalra H, Drummen GP, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci. 2016;17:170.
Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest. 1992;89:1681–4.
Bonetto A, Aydogdu T, Jin X, Zhang Z, Zhan R, Puzis L, et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am J Physiol Endocrinol Metab. 2012;303:E410–21.
Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16:153–66.
Sun L, Quan XQ, Yu S. An Epidemiological survey of cachexia in advanced cancer patients and analysis on its diagnostic and treatment status. Nutr Cancer. 2015;67:1056–62.
Lu S, Li Y, Shen Q, Zhang W, Gu X, Ma M, et al. Carnosol and its analogues attenuate muscle atrophy and fat lipolysis induced by cancer cachexia. J Cachexia Sarcopenia Muscle. 2021;12:779–95.
Wu Q, Sun S, Li Z, Yang Q, Li B, Zhu S, et al. Tumour-originated exosomal miR-155 triggers cancer-associated cachexia to promote tumour progression. Mol Cancer. 2018;17:155.
Wu Q, Sun S, Li Z, Yang Q, Li B, Zhu S, et al. Breast cancer-released exosomes trigger cancer-associated cachexia to promote tumor progression. Adipocyte. 2019;8:31–45.
Zhang G, Liu Z, Ding H, Zhou Y, Doan HA, Sin KWT, et al. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat Commun. 2017;8:589.
Kim NH, Sung NJ, Youn HS, Park SA. Gremlin-1 activates Akt/STAT3 signaling, which increases the glycolysis rate in breast cancer cells. Biochem Biophys Res Commun. 2020;533:1378–84.
Bi YH, Han WQ, Li RF, Wang YJ, Du ZS, Wang XJ, et al. Signal transducer and activator of transcription 3 promotes the Warburg effect possibly by inducing pyruvate kinase M2 phosphorylation in liver precancerous lesions. World J Gastroenterol. 2019;25:1936–49.
Zhao W, Guo W, Zhou Q, Ma SN, Wang R, Qiu Y, et al. In vitro antimetastatic effect of phosphatidylinositol 3-kinase inhibitor ZSTK474 on prostate cancer PC3 cells. Int J Mol Sci. 2013;14:13577–91.
Marrocco I, Altieri F, Rubini E, Paglia G, Chichiarelli S, Giamogante F, et al. Shmt2: a Stat3 signaling new player in prostate cancer energy metabolism. Cells. 2019;8:1048.
Demaria M, Poli V. PKM2, STAT3 and HIF-1α: the Warburg’s vicious circle. Jakstat. 2012;1:194–6.
Vallee A, Guillevin R, Vallee JN. Vasculogenesis and angiogenesis initiation under normoxic conditions through Wnt/beta-catenin pathway in gliomas. Rev Neurosci. 2018;29:71–91.
Yang M, Wang L, Wang X, Wang X, Yang Z, Li J. IL-6 promotes FSH-induced VEGF expression through JAK/STAT3 signaling pathway in bovine granulosa cells. Cell Physiol Biochem. 2017;44:293–302.
Pawlus MR, Wang L, Murakami A, Dai G, Hu CJ. STAT3 or USF2 contributes to HIF target gene specificity. PLoS One. 2013;8:e72358.
Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014–20.
Chaneton B, Gottlieb E. Rocking cell metabolism: revised functions of the key glycolytic regulator PKM2 in cancer. Trends Biochem Sci. 2012;37:309–16.
Puckett DL, Alquraishi M, Chowanadisai W, Bettaieb A. The role of PKM2 in metabolic reprogramming: insights into the regulatory roles of non-coding RNAs. Int J Mol Sci.2021;22:1171.
Vaupel P, Multhoff G. Revisiting the Warburg effect: historical dogma versus current understanding. J Physiol. 2021;599:1745–57.
Zahra K, Dey T, Ashish, Mishra SP, Pandey U. Pyruvate Kinase M2 and cancer: the role of PKM2 in promoting tumorigenesis. Front Oncol. 2020;10:159.
Palsson-McDermott EM, O’Neill LA. The Warburg effect then and now: from cancer to inflammatory diseases. Bioessays. 2013;35:965–73.
Yoon YJ, Kim YH, Jin Y, Chi SW, Moon JH, Han DC, et al. 2’-hydroxycinnamaldehyde inhibits cancer cell proliferation and tumor growth by targeting the pyruvate kinase M2. Cancer Lett. 2018;434:42–55.
Li Y, Wang Y, Liu Z, Guo X, Miao Z, Ma S. Atractylenolide I induces apoptosis and suppresses glycolysis by blocking the JAK2/STAT3 signaling pathway in colorectal cancer cells. Front Pharm. 2020;11:273.
Zhang Q, Liu Q, Zheng S, Liu T, Yang L, Han X, et al. Shikonin inhibits tumor growth of ESCC by suppressing PKM2 mediated aerobic glycolysis and STAT3 phosphorylation. J Cancer. 2021;12:4830–40.
Gao S, Chen M, Wei W, Zhang X, Zhang M, Yao Y, et al. Crosstalk of mTOR/PKM2 and STAT3/c-Myc signaling pathways regulate the energy metabolism and acidic microenvironment of gastric cancer. J Cell Biochem. 2018;120:1193–1202.
Gao X, Wang H, Yang JJ, Liu X, Liu ZR. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol Cell. 2012;45:598–609.
Shirai T, Nazarewicz RR, Wallis BB, Yanes RE, Watanabe R, Hilhorst M, et al. The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J Exp Med. 2016;213:337–54.
Bian Z, Zhang J, Li M, Feng Y, Wang X, Zhang J, et al. LncRNA-FEZF1-AS1 Promotes tumor proliferation and metastasis in colorectal cancer by regulating PKM2 signaling. Clin Cancer Res. 2018;24:4808–19.
Damasceno LEA, Prado DS, Veras FP, Fonseca MM, Toller-Kawahisa JE, Rosa MH, et al. PKM2 promotes Th17 cell differentiation and autoimmune inflammation by fine-tuning STAT3 activation. J Exp Med. 2020;217:e20190613.
Ou B, Sun H, Zhao J, Xu Z, Liu Y, Feng H, et al. Polo-like kinase 3 inhibits glucose metabolism in colorectal cancer by targeting HSP90/STAT3/HK2 signaling. J Exp Clin Cancer Res. 2019;38:426.
Li M, Jin R, Wang W, Zhang T, Sang J, Li N, et al. STAT3 regulates glycolysis via targeting hexokinase 2 in hepatocellular carcinoma cells. Oncotarget. 2017;8:24777–84.
Galli T, Zahraoui A, Vaidyanathan VV, Raposo G, Tian JM, Karin M, et al. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol Biol Cell. 1998;9:1437–48.
Puri N, Roche PA. Ternary SNARE complexes are enriched in lipid rafts during mast cell exocytosis. Traffic. 2006;7:1482–94.
Redell MS, Ruiz MJ, Alonzo TA, Gerbing RB, Tweardy DJ. Stat3 signaling in acute myeloid leukemia: ligand-dependent and -independent activation and induction of apoptosis by a novel small-molecule Stat3 inhibitor. Blood. 2011;117:5701–9.
Zhu B, Zhang QL, Hua JW, Cheng WL, Qin LP. The traditional uses, phytochemistry, and pharmacology of Atractylodes macrocephala Koidz.: a review. J Ethnopharmacol. 2018;226:143–67.
Acknowledgements
This work was supported by National Nature Science Foundation of China (No. 81872496 and 81873056), and the Science and Technology Commission of Shanghai Municipality (20S11902200 and 16DZ2280100).
Author information
Authors and Affiliations
Contributions
MF performed the experiments, analyzed the data, and drafted the manuscript. WS, XG, SL, and QS were involved in performing the experiments. XL and XZ supervised the project, designed the study, and revised the manuscript. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Fan, M., Sun, W., Gu, X. et al. The critical role of STAT3 in biogenesis of tumor-derived exosomes with potency of inducing cancer cachexia in vitro and in vivo. Oncogene 41, 1050–1062 (2022). https://doi.org/10.1038/s41388-021-02151-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-02151-3
This article is cited by
-
Cancer-associated cachexia — understanding the tumour macroenvironment and microenvironment to improve management
Nature Reviews Clinical Oncology (2023)
-
The potential of traditional herbal active ingredients in the treatment of sarcopenia animal models: focus on therapeutic effects and mechanisms
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer
Molecular Cancer (2022)