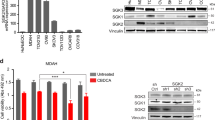
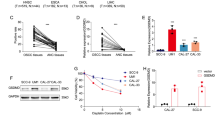
Abstract
Platinum resistance accounts for much of the high mortality and morbidity associated with ovarian cancer. Identification of targets with significant clinical translational potential remains an unmet challenge. Through a high-throughput synthetical lethal screening for clinically relevant targets using 290 kinase inhibitors, we identify calcium/calmodulin-dependent protein kinase II gamma (CAMK2G) as a critical vulnerability in cisplatin-resistant ovarian cancer cells. Pharmacologic inhibition of CAMK2G significantly sensitizes ovarian cancer cells to cisplatin treatment in vitro and in vivo. Mechanistically, CAMK2G directly senses ROS, both basal and cisplatin-induced, to control the phosphorylation of ITPKB at serine 174, which directly regulates ITPKB activity to modulate cisplatin-induced ROS stress. Thereby, CAMK2G facilitates the adaptive redox homeostasis upon cisplatin treatment and drives cisplatin resistance. Clinically, upregulation of CAMK2G activity and ITPKB pS174 correlates with cisplatin resistance in human ovarian cancers. This study reveals a key kinase network consisting of CAMK2G and ITPKB for ROS sense and scavenging in ovarian cancer cells to maintain redox homeostasis, offering a potential strategy for cisplatin resistance treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33.
Holmes D. The problem with platinum. Nature. 2015;527:S218–219.
Holmes D. Ovarian cancer: beyond resistance. Nature. 2015;527:S217.
Wojtaszek JL, Chatterjee N, Najeeb J, Ramos A, Lee M, Bian K, et al. A small molecule targeting mutagenic translesion synthesis improves chemotherapy. Cell. 2019;178:152–159 e111.
Li J, Pan C, Boese AC, Kang J, Umano AD, Magliocca KR, et al. DGKA provides platinum resistance in ovarian cancer through activation of c-JUN-WEE1 signaling. Clin Cancer Res. 2020;26:3843–55.
Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31:1869–83.
Ishida S, McCormick F, Smith-McCune K, Hanahan D. Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell. 2010;17:574–83.
He YJ, Meghani K, Caron MC, Yang C, Ronato DA, Bian J, et al. DYNLL1 binds to MRE11 to limit DNA end resection in BRCA1-deficient cells. Nature. 2018;563:522–6.
Liu D, Zhang XX, Li MC, Cao CH, Wan DY, Xi BX, et al. C/EBPbeta enhances platinum resistance of ovarian cancer cells by reprogramming H3K79 methylation. Nat Commun. 2018;9:1739.
Jin L, Chun J, Pan C, Li D, Lin R, Alesi GN, et al. MAST1 drives cisplatin resistance in human cancers by rewiring cRaf-independent MEK activation. Cancer Cell. 2018;34:315–330 e317.
Pan C, Jin L, Wang X, Li Y, Chun J, Boese AC, et al. Inositol-triphosphate 3-kinase B confers cisplatin resistance by regulating NOX4-dependent redox balance. J Clin Invest. 2019;129:2431–45.
Ferguson FM, Gray NS. Kinase inhibitors: the road ahead. Nat Rev Drug Disco. 2018;17:353–77.
Bhullar KS, Lagaron NO, McGowan EM, Parmar I, Jha A, Hubbard BP, et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer. 2018;17:48.
Pouillon V, Hascakova-Bartova R, Pajak B, Adam E, Bex F, Dewaste V, et al. Inositol 1,3,4,5-tetrakisphosphate is essential for T lymphocyte development. Nat Immunol. 2003;4:1136–43.
Miller AT, Beisner DR, Liu D, Cooke MP. Inositol 1,4,5-trisphosphate 3-kinase B is a negative regulator of BCR signaling that controls B cell selection and tolerance induction. J Immunol. 2009;182:4696–704.
Marechal Y, Queant S, Polizzi S, Pouillon V, Schurmans S. Inositol 1,4,5-trisphosphate 3-kinase B controls survival and prevents anergy in B cells. Immunobiology. 2011;216:103–9.
Hook SS, Means AR. Ca(2+)/CaM-dependent kinases: from activation to function. Annu Rev Pharm Toxicol. 2001;41:471–505.
Gu Y, Zhang J, Ma X, Kim BW, Wang H, Li J, et al. Stabilization of the c-Myc protein by CAMKIIgamma promotes T cell lymphoma. Cancer Cell. 2017;32:115–128 e117.
Hegyi B, Fasoli A, Ko CY, Van BW, Alim CC, Shen EY, et al. CaMKII serine 280 O-GlcNAcylation links diabetic hyperglycemia to proarrhythmia. Circ Res. 2021;129:98–113.
Liu X, Wang S, Guo X, Li Y, Ogurlu R, Lu F. et al. Increased ROS-mediated CaMKII activation contributes to calcium handling abnormalities and impaired contraction in barth syndrome. Circulation. 2021;143:1894–1911.
Pellicena P, Schulman H. CaMKII inhibitors: from research tools to therapeutic agents. Front Pharm. 2014;5:21.
Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–50.
Sanij E, Hannan KM, Xuan J, Yan S, Ahern JE, Trigos AS, et al. CX-5461 activates the DNA damage response and demonstrates therapeutic efficacy in high-grade serous ovarian cancer. Nat Commun. 2020;11:2641.
Schroyer AL, Stimes NW, Abi Saab WF, Chadee DN. MLK3 phosphorylation by ERK1/2 is required for oxidative stress-induced invasion of colorectal cancer cells. Oncogene. 2018;37:1031–40.
Wen C, Wang H, Wu X, He L, Zhou Q, Wang F, et al. ROS-mediated inactivation of the PI3K/AKT pathway is involved in the antigastric cancer effects of thioredoxin reductase-1 inhibitor chaetocin. Cell Death Dis. 2019;10:809.
Chen D, Xia S, Wang M, Lin R, Li Y, Mao H, et al. Mutant and wild-type isocitrate dehydrogenase 1 share enhancing mechanisms involving distinct tyrosine kinase cascades in cancer. Cancer Disco. 2019;9:756–77.
Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, et al. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci Signal. 2009;2:ra73.
Domcke S, Sinha R, Levine DA, Sander C, Schultz N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat Commun. 2013;4:2126.
Nassal D, Gratz D, Hund TJ. Challenges and opportunities for therapeutic targeting of calmodulin kinase ii in heart. Front Pharm. 2020;11:35.
Acknowledgements
This work was supported by the grants from the National Natural Science Foundation of China 82003194 (CP) and 82073074 (BL), Guangdong Basic and Applied Basic Research Foundation 2021A1515012446 (CP), Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation 2020B1212060018 (CP), and 2019 AACR Anna D. Barker Basic Cancer Research Fellowship (CP).
Author information
Authors and Affiliations
Contributions
JL: experimental design, data acquisition, data analysis, manuscript writing. CZ, MW, QD, QY, XY, JL, WP: experimental methods, data acquisition. CZh, HH: key reagents. AU: manuscript editing. SY, BL: data analysis, funding. CP: funding, supervision, experimental design, data analysis, manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, J., Zheng, C., Wang, M. et al. ROS-regulated phosphorylation of ITPKB by CAMK2G drives cisplatin resistance in ovarian cancer. Oncogene 41, 1114–1128 (2022). https://doi.org/10.1038/s41388-021-02149-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-02149-x
This article is cited by
-
Suppression of ITPKB degradation by Trim25 confers TMZ resistance in glioblastoma through ROS homeostasis
Signal Transduction and Targeted Therapy (2024)
-
TNFAIP2 confers cisplatin resistance in head and neck squamous cell carcinoma via KEAP1/NRF2 signaling
Journal of Experimental & Clinical Cancer Research (2023)
-
ROS-mediated SRMS activation confers platinum resistance in ovarian cancer
Oncogene (2023)