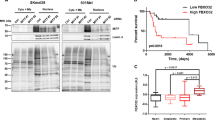
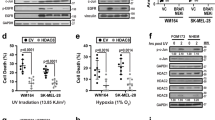
Abstract
Melanoma is a type of skin cancer that develops in pigment-producing melanocytes and often spreads to other parts of the body. Aberrant gene expression has been considered as a crucial step for increasing the risk of melanomagenesis, but how chromatin reorganization contributes to this pathogenic process is still not well understood. Here we report that matrix metalloproteinase 9 (MMP-9) localizes to the nucleus of melanoma cells and potentiates gene expression by proteolytically clipping the histone H3 N-terminal tail (H3NT). From genome-wide studies, we discovered that growth-regulatory genes are selectively targeted and activated by MMP-9-dependent H3NT proteolysis in melanoma cells. MMP-9 cooperates functionally with p300/CBP because MMP-9 cleaves H3NT in a manner that is dependent on p300/CBP-mediated acetylation of H3K18. The functional significance of MMP-9-dependent H3NT proteolysis is further underscored by the fact that RNAi knockdown and small-molecule inhibition of MMP-9 and p300/CBP impede melanomagenic gene expression and melanoma tumor growth. Together, our data establish new functions and mechanisms for nuclear MMP-9 in promoting melanomagenesis and demonstrate how MMP-9-dependent H3NT proteolysis can be exploited to prevent and treat melanoma skin cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Boissy RE, Nordlund JJ. Molecular basis of congenital hypopigmentary disorders in humans: a review. Pigment Cell Res. 1997;10:12–24.
Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nat. 2007;445:851–7.
Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiological Rev. 2004;84:1155–228.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54.
Dong J, Phelps RG, Qiao R, Yao S, Benard O, Ronai Z, et al. BRAF oncogenic mutations correlate with progression rather than initiation of human melanoma. Cancer Res. 2003;63:3883–5.
Dumaz N, Hayward R, Martin J, Ogilvie L, Hedley D, Curtin JA, et al. In melanoma, RAS mutations are accompanied by switching signaling from BRAF to CRAF and disrupted cyclic AMP signaling. Cancer Res. 2006;66:9483–91.
Krauthammer M, Kong Y, Bacchiocchi A, Evans P, Pornputtapong N, Wu C, et al. Exome sequencing identifies recurrent mutations in NF1 and RASopathy genes in sun-exposed melanomas. Nat Genet. 2015;47:996–1002.
Chen H, Weng QY, Fisher DE. UV signaling pathways within the skin. J Investig Dermatol. 2014;134:2080–5.
Fu C, Chen J, Lu J, Yi L, Tong X, Kang L, et al. Roles of inflammation factors in melanogenesis (Review). Mol Med Rep. 2020;21:1421–30.
Körner A, Pawelek J. Mammalian tyrosinase catalyzes three reactions in the biosynthesis of melanin. Science. 1982;217:1163–5.
Zhou S, Zeng H, Huang J, Lei L, Tong X, Li S, et al. Epigenetic regulation of melanogenesis. Ageing Res Rev. 2021;69:101349.
Hoffmann F, Niebel D, Aymans P, Ferring-Schmitt S, Dietrich D. Landsberg J. H3K27me3 and EZH2 expression in melanoma: relevance for melanoma progression and response to immune checkpoint blockade. Clin Epigenet. 2020;12:24.
Mahmoud F, Shields B, Makhoul I, Hutchins LF, Shalin SC, Tackett AJ. Role of EZH2 histone methyltrasferase in melanoma progression and metastasis. Cancer Biol Ther. 2016;17:579–91.
Faião-Flores F, Emmons MF, Durante MA, Kinose F, Saha B, Fang B, et al. HDAC inhibition enhances the in vivo efficacy of MEK inhibitor therapy in uveal melanoma. Clin Cancer Res. 2019;25:5686–701.
Wilmott JS, Colebatch AJ, Kakavand H, Shang P, Carlino MS, Thompson JF, et al. Expression of the class 1 histone deacetylases HDAC8 and 3 are associated with improved survival of patients with metastatic melanoma. Mod Pathol. 2015;28:884–94.
Lue JK, Prabhu SA, Liu Y, Gonzalez Y, Verma A, Mundi PS, et al. Precision targeting with EZH2 and HDAC inhibitors in epigenetically dysregulated lymphomas. Clin Cancer Res. 2019;25:5271–83.
Zhang X, Zhao X, Fiskus W, Lin J, Lwin T, Rao R, et al. Coordinated silencing of MYC-mediated miR-29 by HDAC3 and EZH2 as a therapeutic target of histone modification in aggressive B-Cell lymphomas. Cancer Cell. 2012;22:506–23.
Agrawal A, Romero-Perez D, Jacobsen JA, Villarreal FJ, Cohen SM. Zinc-binding groups modulate selective inhibition of MMPs. ChemMedChem. 2008;3:812–20.
Bruschi F, Pinto B. The significance of matrix metalloproteinases in parasitic infections involving the central nervous system. Pathogens. 2013;2:105–29.
Klein T, Bischoff R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 2011;41:271–90.
Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–73.
Santos MC, de Souza AP, Gerlach RF, Trevilatto PC, Scarel-Caminaga RM, Line SR. Inhibition of human pulpal gelatinases (MMP-2 and MMP-9) by zinc oxide cements. J Oral Rehabil. 2004;31:660–4.
Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9). Crit Rev Biochem Mol Biol. 2002;37:375–536.
Vandooren J, Van den Steen PE, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit Rev Biochem Mol Biol. 2013;48:222–72.
Kim K, Shin Y, Kim J, Ulmer TS, An W. H3K27me1 is essential for MMP-9-dependent H3N-terminal tail proteolysis during osteoclastogenesis. Epigenetics Chromatin. 2018;11:23.
Papazafiropoulou A, Tentolouris N. Matrix metalloproteinases and cardiovascular diseases. Hippokratia. 2009;13:76–82.
Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–44.
Birkedal-Hansen H. Proteolytic remodeling of extracellular matrix. Curr Opin Cell Biol. 1995;7:728–35.
Vargová V, Pytliak M, Mechírová V. Matrix metalloproteinases. Experientia Supplementum (2012). 2012;103:1–33.
Kim K, Punj V, Kim JM, Lee S, Ulmer TS, Lu W, et al. MMP-9 facilitates selective proteolysis of the histone H3 tail at genes necessary for proficient osteoclastogenesis. Genes Dev. 2016;30:208–19.
Shin Y, Ghate NB, Moon B, Park K, Lu W, An W. DNMT and HDAC inhibitors modulate MMP-9-dependent H3 N-terminal tail proteolysis and osteoclastogenesis. Epigenetics Chromatin. 2019;12:25.
Guarneri C, Bevelacqua V, Polesel J, Falzone L, Cannavò PS, Spandidos DA, et al. NF‑κB inhibition is associated with OPN/MMP‑9 downregulation in cutaneous melanoma. Oncol Rep. 2017;37:737–46.
Napoli S, Scuderi C, Gattuso G, Bella VD, Candido S, Basile MS, et al. Functional roles of matrix metalloproteinases and their inhibitors in melanoma. Cells. 2020;9:1151.
Rangaswami H, Kundu GC. Osteopontin stimulates melanoma growth and lung metastasis through NIK/MEKK1-dependent MMP-9 activation pathways. Oncol Rep. 2007;18:909–15.
Ding L, Wen Y, Zhang X, Zhao F, Lv K, Shi J-H, et al. Transcriptional network constituted of CBP, Ku70, NOX2, and BAX prevents the cell death of necrosis, paraptosis, and apoptosis in human melanoma. Cell Death Discov. 2021;7:40.
Lin WM, Baker AC, Beroukhim R, Winckler W, Feng W, Marmion JM, et al. Modeling genomic diversity and tumor dependency in malignant melanoma. Cancer Res. 2008;68:664–73.
Rice JC, Weekley BH, Kanholm T, Chen Z, Lee S, Fernandez DJ, et al. MMP-2 is a novel histone H3 N-terminal protease necessary for myogenic gene activation. Epigenetics Chromatin. 2021;14:23.
Daura E, Tegelberg S, Yoshihara M, Jackson C, Simonetti F, Aksentjeff K, et al. Cystatin B-deficiency triggers ectopic histone H3 tail cleavage during neurogenesis. Neurobiol Dis. 2021;156:105418.
Ferrari KJ, Amato S, Noberini R, Toscani C, Fernández-Pérez D, Rossi A, et al. Intestinal differentiation involves cleavage of histone H3 N-terminal tails by multiple proteases. Nucleic Acids Res. 2021;49:791–804.
Shen J, Xiang X, Chen L, Wang H, Wu L, Sun Y, et al. JMJD5 cleaves monomethylated histone H3 N-tail under DNA damaging stress. EMBO Rep. 2017;18:2131–43.
Kim E, Zucconi BE, Wu M, Nocco SE, Meyers DJ, McGee JS, et al. MITF expression predicts therapeutic vulnerability to p300 inhibition in human melanoma. Cancer Res. 2019;79:2649–61.
Wang R, He Y, Robinson V, Yang Z, Hessler P, Lasko LM, et al. Targeting lineage-specific MITF pathway in human melanoma cell lines by A-485, the selective small-molecule inhibitor of p300/CBP. Mol Cancer Therapeutics. 2018;17:2543–50.
Kim JM, Shin Y, Lee S, Kim MY, Punj V, Shin HI, et al. MacroH2A1.2 inhibits prostate cancer-induced osteoclastogenesis through cooperation with HP1α and H1.2. Oncogene. 2018;37:5749–65.
Frankish A, Diekhans M, Ferreira A-M, Johnson R, Jungreis I, Loveland J, et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019;47:D766–D773.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21.
Bähler J, Zhao X, Valen E, Parker BJ, Sandelin A. Systematic clustering of transcription start site landscapes. PLoS ONE. 2011;6:e23409.
Kim K, Kim JM, Kim JS, Choi J, Lee YS, Neamati N, et al. VprBP has intrinsic kinase activity targeting histone H2A and represses gene transcription. Mol Cell. 2013;52:459–67.
Author contribution
YS designed the experiments. WA provided guidance throughout. YS, SK, NG, SR, and WA performed experiments and analyzed data. YS and WA wrote the manuscript.
Funding
This work was supported by NIH Grant AR073233 awarded to WA. The study was also partly supported by award number P30CA014089 from the National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Shin, Y., Kim, S., Ghate, N.B. et al. MMP-9 drives the melanomagenic transcription program through histone H3 tail proteolysis. Oncogene 41, 560–570 (2022). https://doi.org/10.1038/s41388-021-02109-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-02109-5
This article is cited by
-
The MMP-2 histone H3 N-terminal tail protease is selectively targeted to the transcription start sites of active genes
Epigenetics & Chromatin (2023)
-
The micronuclear histone H3 clipping in the unicellular eukaryote Tetrahymena thermophila
Marine Life Science & Technology (2022)