Abstract
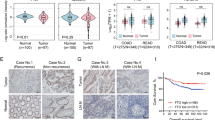
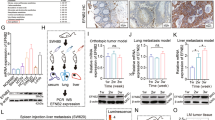
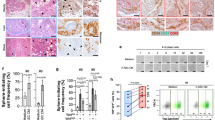
Low levels of ITLN1 have been correlated with obesity-related colorectal carcinogenesis, however, the specific functions and underlying mechanisms remain unclear. Thus, we sought to explore the inhibitory role of ITLN1 in the tumor-permissive microenvironment that exists during the first occurrence and subsequent development of colorectal carcinoma (CRC). Results indicated that ITLN1 was frequently lost in CRC tissues and ITLN1 to be an independent prognostic predictor of CRC. Orthotopic and subcutaneous tumor xenograft approaches were then used to further confirm the protective role of ITLN1 during tumor progression. Increased ITLN1 expression in CRC cells significantly inhibited local pre-existing vessels sprouting, EPC recruitment and the infiltration of immunosuppressive myeloid-derived suppressor cells (MDSCs) into tumor tissues without affecting the behavior of CRC cells in vitro. Comparatively, ITLN1-derived MDSCs had a lower suppressive effect on T cell proliferation, NOS2 expression, and ROS production. In addition, ITLN1 overexpression markedly suppressed bone marrow (BM)-derived hematopoietic progenitor cells (HPC) differentiation into MDSCs as well as NOS2 activity on MDSCs. Using H-2b+YFP + chimerism through bone marrow transplantation, increased ITLN1 in HCT116 significantly reduced the BM-derived EPCs and MDSCs in vivo mobilization. Mechanistically, results indicated ITLN1 inhibited tumor-derived IL-17D and CXCL2 (MIP2) through the KEAP1/Nrf2/ROS/IL-17D and p65 NF-ĸB/CXCL2 signaling cascades dependent on PI3K/AKT/GSK3ß. This effect was reversed by the PI3K selective inhibitor LY294002. Collectively, ITLN1 synergistically suppressed IL-17D and CXCL2-mediated tumor vascularization, bone marrow derived EPC recruitment, as well as MDSCs generation and trafficking. Thus, ITLN1 potentially serves as a critical prognostic and therapeutic target for CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin. 2018;68:394–424.
Bailey MH, Tokheim C, Porta-Pardo E, Sengupta S, Bertrand D, Weerasinghe A, et al. Comprehensive characterization of cancer driver genes and mutations. Cell. 2018;174:1034–5.
Paul J, Soujon M, Wengner AM, Zitzmann-Kolbe S, Sturz A, Haike K, et al. Simultaneous inhibition of PI3Kdelta and PI3Kalpha induces ABC-DLBCL regression by blocking BCR-dependent and -independent activation of NF-kappaB and AKT. Cancer Cell. 2017;31:64–78.
Hempen PM, Zhang L, Bansal RK, Iacobuzio-Donahue CA, Murphy KM, Maitra A, et al. Evidence of selection for clones having genetic inactivation of the activin A type II receptor (ACVR2) gene in gastrointestinal cancers. Cancer Res. 2003;63:994–9.
Terzić J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–.e2105.
Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74.
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68.
Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer cell. 2004;6:409–21.
Bronte V, Brandau S, Chen S-H, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016;7:12150.
Katoh H, Wang D, Daikoku T, Sun H, Dey SK, Dubois RN. CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell. 2013;24:631–44.
Tanigawa N, Amaya H, Matsumura M, Lu C, Kitaoka A, Matsuyama K, et al. Tumor angiogenesis and mode of metastasis in patients with colorectal cancer. Cancer Res. 1997;57:1043–6.
Tanigawa N, Amaya H, Matsumura M, Shimomatsuya T, Horiuchi T, Muraoka R, et al. Extent of tumor vascularization correlates with prognosis and hematogenous metastasis in gastric carcinomas. Cancer Res. 1996;56:2671–6.
Xu H, Zhang Y, Pena MM, Pirisi L, Creek KE. Six1 promotes colorectal cancer growth and metastasis by stimulating angiogenesis and recruiting tumor-associated macrophages. Carcinogenesis. 2017;38:281–92.
Zhang C, Zhou C, Wu XJ, Yang M, Yang ZH, Xiong HZ, et al. Human CD133-positive hematopoietic progenitor cells initiate growth and metastasis of colorectal cancer cells. Carcinogenesis. 2014;35:2771–7.
Mody K, Baldeo C, Bekaii-Saab T. Antiangiogenic therapy in colorectal cancer. Cancer J (Sudbury, Mass). 2018;24:165–70.
Lee JK, Schnee J, Pang M, Wolfert M, Baum LG, Moremen KW, et al. Human homologs of the Xenopus oocyte cortical granule lectin XL35. Glycobiology. 2001;11:65–73.
Tsuji S, Uehori J, Matsumoto M, Suzuki Y, Matsuhisa A, Toyoshima K, et al. Human intelectin is a novel soluble lectin that recognizes galactofuranose in carbohydrate chains of bacterial cell wall. J Biol Chem. 2001;276:23456–63.
Mancinelli R, Olivero F, Carpino G, Overi D, Rosa L, Lepanto MS, et al. Role of lactoferrin and its receptors on biliary epithelium. Biometals. 2018;31:369–79.
Shin K, Wakabayashi H, Yamauchi K, Yaeshima T, Iwatsuki K. Recombinant human intelectin binds bovine lactoferrin and its peptides. Biol Pharm Bull. 2008;31:1605–8.
Suzuki YA, Lopez V, Lonnerdal B. Mammalian lactoferrin receptors: structure and function. Cell Mol life Sci: CMLS. 2005;62:2560–75.
Yang RZ, Lee MJ, Hu H, Pray J, Wu HB, Hansen BC, et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290:E1253–1261.
Alissa EM, Al-Salmi MM, Alama NA, Ferns GA. Role of omentin-1 and C-reactive protein in obese subjects with subclinical inflammation. J Clin Transl Endocrinol. 2016;3:7–11.
Washimi K, Yokose T, Yamashita M, Kageyama T, Suzuki K, Yoshihara M, et al. Specific expression of human intelectin-1 in malignant pleural mesothelioma and gastrointestinal goblet cells. PloS One. 2012;7:e39889.
Wali A, Morin PJ, Hough CD, Lonardo F, Seya T, Carbone M, et al. Identification of intelectin overexpression in malignant pleural mesothelioma by serial analysis of gene expression (SAGE). Lung Cancer. 2005;48:19–29.
Li D, Mei H, Pu J, Xiang X, Zhao X, Qu H, et al. Intelectin 1 suppresses the growth, invasion and metastasis of neuroblastoma cells through up-regulation of N-myc downstream regulated gene 2. Mol Cancer. 2015;14:47.
Au-Yeung CL, Yeung TL, Achreja A, Zhao H, Yip KP, Kwan SY, et al. ITLN1 modulates invasive potential and metabolic reprogramming of ovarian cancer cells in omental microenvironment. Nat Commun. 2020;11:3546.
Zhang YY, Zhou LM. Omentin-1, a new adipokine, promotes apoptosis through regulating Sirt1-dependent p53 deacetylation in hepatocellular carcinoma cells. Eur J Pharmacol. 2013;698:137–44.
Li D, Zhao X, Xiao Y, Mei H, Pu J, Xiang X, et al. Intelectin 1 suppresses tumor progression and is associated with improved survival in gastric cancer. Oncotarget. 2015;6:16168–82.
Maeda K, Saigo C, Kito Y, Sakuratani T, Yoshida K, Takeuchi T. Expression of TMEM207 in colorectal cancer: relation between TMEM207 and Intelectin-1. J Cancer. 2016;7:207–13.
Kim HJ, Kang UB, Lee H, Jung JH, Lee ST, Yu MH, et al. Profiling of differentially expressed proteins in stage IV colorectal cancers with good and poor outcomes. J Proteom. 2012;75:2983–97.
Wang D, Sun H, Wei J, Cen B, DuBois RN. CXCL1 is critical for premetastatic niche formation and metastasis in colorectal cancer. Cancer Res. 2017;77:3655–65.
Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25.
Hanrahan V, Currie MJ, Gunningham SP, Morrin HR, Scott PA, Robinson BA, et al. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma-carcinoma sequence during colorectal cancer progression. J Pathol. 2003;200:183–94.
Caiado F, Dias S. Endothelial progenitor cells and integrins: adhesive needs. Fibrogenes Tissue Repair. 2012;5:4.
Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–8.
Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–201.
Patan S. Vasculogenesis and angiogenesis. Cancer Treat Res. 2004;117:3–32.
Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307.
Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Investig. 1999;103:1231–6.
Starnes T, Broxmeyer HE, Robertson MJ, Hromas R. Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. J Immunol. 2002;169:642–6.
Kaplan RN, Psaila B, Lyden D. Niche-to-niche migration of bone-marrow-derived cells. Trends Mol Med. 2007;13:72–81.
Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, et al. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood. 2005;105:1068–77.
Jaramillo MC, Zhang DD. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–91.
Taki M, Abiko K, Baba T, Hamanishi J, Yamaguchi K, Murakami R, et al. Snail promotes ovarian cancer progression by recruiting myeloid-derived suppressor cells via CXCR2 ligand upregulation. Nat Commun. 2018;9:1685.
Zhang H, Ye YL, Li MX, Ye SB, Huang WR, Cai TT, et al. CXCL2/MIF-CXCR2 signaling promotes the recruitment of myeloid-derived suppressor cells and is correlated with prognosis in bladder cancer. Oncogene. 2017;36:2095–104.
Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD. Nrf2 is controlled by two distinct beta-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–81.
Gonzalez-Donquiles C, Alonso-Molero J, Fernandez-Villa T, Vilorio-Marques L, Molina AJ, Martin V. The NRF2 transcription factor plays a dual role in colorectal cancer: A systematic review. PloS one. 2017;12:e0177549.
Li CQ, Kim MY, Godoy LC, Thiantanawat A, Trudel LJ, Wogan GN. Nitric oxide activation of Keap1/Nrf2 signaling in human colon carcinoma cells. Proc Natl Acad Sci USA. 2009;106:14547–51.
Huang Y, Mao Y, Li H, Shen G, Nan G. Knockdown of Nrf2 inhibits angiogenesis by downregulating VEGF expression through PI3K/Akt signaling pathway in cerebral microvascular endothelial cells under hypoxic conditions. Biochem Cell Biol. 2018;96:475–82.
Kim TH, Hur EG, Kang SJ, Kim JA, Thapa D, Lee YM, et al. NRF2 blockade suppresses colon tumor angiogenesis by inhibiting hypoxia-induced activation of HIF-1alpha. Cancer Res. 2011;71:2260–75.
Saddawi-Konefka R, Seelige R, Gross ET, Levy E, Searles SC, Washington A Jr., et al. Nrf2 induces IL-17D to mediate tumor and virus surveillance. Cell Rep. 2016;16:2348–58.
Schmidt A, Brixius K, Bloch W. Endothelial precursor cell migration during vasculogenesis. Circulation Res. 2007;101:125–36.
Liu Y, Cao X. Characteristics and significance of the pre-metastatic niche. Cancer cell. 2016;30:668–81.
Achyut BR, Shankar A, Iskander AS, Ara R, Angara K, Zeng P, et al. Bone marrow derived myeloid cells orchestrate antiangiogenic resistance in glioblastoma through coordinated molecular networks. Cancer Lett. 2015;369:416–26.
Komiya T, Tanigawa Y, Hirohashi S. Cloning of the novel gene intelectin, which is expressed in intestinal paneth cells in mice. Biochemical Biophys Res Commun. 1998;251:759–62.
Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013;19:1114–23.
Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–7.
Morandi F, Di Carlo E, Ferrone S, Petretto A, Pistoia V, Airoldi I. IL-27 in human secondary lymphoid organs attracts myeloid dendritic cells and impairs HLA class I-restricted antigen presentation. J Immunol. 2014;192:2634–42.
Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–44.
Aleksandrova K, di Giuseppe R, Isermann B, Biemann R, Schulze M, Wittenbecher C, et al. Circulating omentin as a novel biomarker for colorectal cancer risk: data from the EPIC-potsdam cohort study. Cancer Res. 2016;76:3862–71.
Moldogazieva NT, Lutsenko SV Reactive oxygen and nitrogen species-induced protein modifications: implication in carcinogenesis and anticancer therapy. Cancer Res. 2018;78:6040-7.
Xie D, Sham JS, Zeng WF, Lin HL, Che LH, Wu HX, et al. Heterogeneous expression and association of beta-catenin, p16 and c-myc in multistage colorectal tumorigenesis and progression detected by tissue microarray. Int J Cancer. 2003;107:896–902.
Cai MY, Tong ZT, Zheng F, Liao YJ, Wang Y, Rao HL, et al. EZH2 protein: a promising immunomarker for the detection of hepatocellular carcinomas in liver needle biopsies. Gut. 2011;60:967–76.
Acknowledgements
We would appreciate Mr. Junhui Huang from Guangzhou Jiamai Biotechnology co., LTD for helping us analyze FCM data. We would also appreciate Dr Hai-yun Wang and Miss. Meng Zheng from Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center for helping us with the revision.
Funding
This work was supported by grants from the National Key R&D Program of China (No. 2017YFC1309000 and No. 2016YFC1302305); the National Natural Science Foundation of China (No.81430055, 81572359, 81602063, 81730072, 81772595, 8197227); the National Natural Science Foundation for Youth (No.81502259); the Natural Science Foundation of Guangdong (No. S1014030001589); the 61th China Postdoctoral Science foundation (2017M612816) and the Guangzhou Science and Technology Plan Projects (No. 201803040019, 201904020044).
Author information
Authors and Affiliations
Contributions
LC, DX designed the experiments and wrote the manuscript; LC, XHJ, JL, JLD performed the experiments; MYC and JWC collected the surgical sample and performed TMA assay; ZHF ran the TCGA analysis; AMG, FWW helped interpreting the data and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chen, L., Jin, XH., Luo, J. et al. ITLN1 inhibits tumor neovascularization and myeloid derived suppressor cells accumulation in colorectal carcinoma. Oncogene 40, 5925–5937 (2021). https://doi.org/10.1038/s41388-021-01965-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-01965-5