Abstract
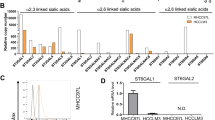
Hepatocellular carcinoma (HCC) is an extremely metastatic tumor. Sialic acids (SAs) are associated with cancer development and metastasis. NEU4 is a sialidase that removes SAs from glycoconjugates, while the function of the NEU4 in HCC has not been clearly explored. In our research, we found the NEU4 expression was significantly down-regulated in HCC tissues, which was correlated with high grades and poor outcomes of HCC. The NEU4 expression could be regulated by histone acetylation. In the functional analysis of NEU4, the cell motility was inhibited when NEU4 was overexpressed, and restored when NEU4 expression was down-regulated. Similarly, NEU4 over-expressed HCC cells showed less metastasis in athymic nude mice. Further study revealed that NEU4 could inhibit cell migration by enzymatic decomposition of SAs. Our results verified a NEU4 active site (NEU4E235) and overexpressing inactivates NEU4E235A that weakens the inhibition ability to cell migration. Further, 70 kinds of specific interacting proteins of NEU4 including CD44 were identified through mass spectrum. Moreover, the α2,3-linked SAs on CD44 were decreased and the hyaluronic acid (HA) binding ability was increased when NEU4 over-expressed or activated. Additionally, the mutation of CD44 with six N-glycosylation sites showed less sensibility to NEU4 on cell migration compared with wild-type CD44. In summary, our results revealed the mechanism of low expression of NEU4 in HCC and its inhibitory effect on cell migration by removal of SAs on CD44, which may provide new treatment strategies to control the motility and metastasis of HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Villanueva A. Hepatocellular Carcinoma. N. Engl J Med. 2019;380:1450–62.
Wu L, Li H, Chen S, Wu X, Chen X, Wang F. Catalpol inhibits the proliferation, migration and metastasis of HCC cells by regulating miR‑140–5p expression. Mol Med Rep. 2021; 23:29.
Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446:1023–9.
Painbeni T, Gamelin E, Cailleux A, Le Bouil A, Boisdron-Celle M, Daver A, et al. Plasma sialic acid as a marker of the effect of the treatment on metastatic colorectal cancer. Eur J Cancer. 1997;33:2216–20.
Laganà A, Pardo-Martínez B, Marino A, Fago G, Bizzarri M. Determination of serum total lipid and free N-acetylneuraminic acid in genitourinary malignancies by fluorimetric high performance liquid chromatography. Relevance of free N-acetylneuraminic acid as tumour marker. Clin Chim Acta. 1995;243:165–79.
Lv J, Lv CQ, Xu L, Yang H. Plasma content variation and correlation of plasmalogen and GIS, TC, and TPL in gastric carcinoma patients: a comparative study. Med Sci Monit Basic Res. 2015;21:157–60.
Lv J, Lv CQ, Mei P, Qi SM. Diagnosis value of membrane glycolipids biochemistry index in intracranial and gastrointestinal tumors. Asian Pac J Cancer Prev. 2015;16:2693–6.
Kongtawelert P, Tangkijvanich P, Ong-Chai S, Poovorawan Y. Role of serum total sialic acid in differentiating cholangiocarcinoma from hepatocellular carcinoma. World J Gastroenterol. 2003;9:2178–81.
Sun H, Zhou Y, Jiang H, Xu Y. Elucidation of functional roles of sialic acids in cancer migration. Front Oncol. 2020;10:401.
Bassagañas S, Pérez-Garay M, Peracaula R. Cell surface sialic acid modulates extracellular matrix adhesion and migration in pancreatic adenocarcinoma cells. Pancreas. 2014;43:109–17.
Angata K, Fukuda M. Roles of polysialic acid in migration and differentiation of neural stem cells. Methods Enzymol. 2010;479:25–36.
Lee M, Lee HJ, Seo WD, Park KH, Lee YS. Sialylation of integrin beta1 is involved in radiation-induced adhesion and migration in human colon cancer cells. Int J Radiat Oncol Biol Phys. 2010;76:1528–36.
Ou L, He X, Liu N, Song Y, Li J, Gao L, et al. Sialylation of FGFR1 by ST6Gal‑I overexpression contributes to ovarian cancer cell migration and chemoresistance. Mol Med Rep. 2020;21:1449–60.
Gong A, Zhao X, Pan Y, Qi Y, Li S, Huang Y, et al. The lncRNA MEG3 mediates renal cell cancer progression by regulating ST3Gal1 transcription and EGFR sialylation. J Cell Sci. 2020; 133: jcs244020.
Birch M, Mitchell S, Hart IR. Isolation and characterization of human melanoma cell variants expressing high and low levels of CD44. Cancer Res. 1991;51:6660–7.
Shiozaki K, Takahashi K, Hosono M, Yamaguchi K, Hata K, Shiozaki M, et al. Phosphatidic acid-mediated activation and translocation to the cell surface of sialidase NEU3, promoting signaling for cell migration. FASEB J. 2015;29:2099–111.
Miyagi T, Yamaguchi K. Mammalian sialidases: physiological and pathological roles in cellular functions. Glycobiology. 2012;22:880–96.
Miyagi T, Wada T, Yamaguchi K, Hata K. Sialidase and malignancy: a minireview. Glycoconj J. 2004;20:189–98.
Miyagi T, Wada T, Yamaguchi K, Shiozaki K, Sato I, Kakugawa Y, et al. Human sialidase as a cancer marker. Proteomics. 2008;8:3303–11.
Comelli EM, Amado M, Lustig SR, Paulson JC. Identification and expression of Neu4, a novel murine sialidase. Gene. 2003;321:155–61.
Seyrantepe V, Landry K, Trudel S, Hassan JA, Morales CR, Pshezhetsky AV. Neu4, a novel human lysosomal lumen sialidase, confers normal phenotype to sialidosis and galactosialidosis cells. J Biol Chem. 2004;279:37021–9.
Takahashi K, Mitoma J, Hosono M, Shiozaki K, Sato C, Yamaguchi K, et al. Sialidase NEU4 hydrolyzes polysialic acids of neural cell adhesion molecules and negatively regulates neurite formation by hippocampal neurons. J Biol Chem. 2012;287:14816–26.
Sorice M, Matarrese P, Tinari A, Giammarioli AM, Garofalo T, Manganelli V, et al. Raft component GD3 associates with tubulin following CD95/Fas ligation. FASEB J. 2009;23:3298–308.
Sano R, Annunziata I, Patterson A, Moshiach S, Gomero E, Opferman J, et al. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol Cell. 2009;36:500–11.
Shiozaki K, Yamaguchi K, Takahashi K, Moriya S, Miyagi T. Regulation of sialyl Lewis antigen expression in colon cancer cells by sialidase NEU4. J Biol Chem. 2011;286:21052–61.
Yamaguchi K, Hata K, Koseki K, Shiozaki K, Akita H, Wada T, et al. Evidence for mitochondrial localization of a novel human sialidase (NEU4). Biochemical J. 2005;390:85–93.
Grinchuk OV, Yenamandra SP, Iyer R, Singh M, Lee HK, Lim KH, et al. Tumor-adjacent tissue co-expression profile analysis reveals pro-oncogenic ribosomal gene signature for prognosis of resectable hepatocellular carcinoma. Mol Oncol. 2018;12:89–113.
Waterhouse A, Bertoni M, Bienert S, Studer G, Tauriello G, Gumienny R, et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–w303.
Uemura T, Shiozaki K, Yamaguchi K, Miyazaki S, Satomi S, Kato K, et al. Contribution of sialidase NEU1 to suppression of metastasis of human colon cancer cells through desialylation of integrin beta4. Oncogene. 2009;28:1218–29.
Hou G, Liu G, Yang Y, Li Y, Yuan S, Zhao L, et al. Neuraminidase 1 (NEU1) promotes proliferation and migration as a diagnostic and prognostic biomarker of hepatocellular carcinoma. Oncotarget. 2016;7:64957–66.
Nath S, Mandal C, Chatterjee U, Mandal C. Association of cytosolic sialidase Neu2 with plasma membrane enhances Fas-mediated apoptosis by impairing PI3K-Akt/mTOR-mediated pathway in pancreatic cancer cells. Cell Death Dis. 2018;9:210.
Ueno S, Saito S, Wada T, Yamaguchi K, Satoh M, Arai Y, et al. Plasma membrane-associated sialidase is up-regulated in renal cell carcinoma and promotes interleukin-6-induced apoptosis suppression and cell motility. J Biol Chem. 2006;281:7756–64.
Yamaguchi K, Hata K, Wada T, Moriya S, Miyagi T. Epidermal growth factor-induced mobilization of a ganglioside-specific sialidase (NEU3) to membrane ruffles. Biochem Biophys Res Commun. 2006;346:484–90.
Yamanami H, Shiozaki K, Wada T, Yamaguchi K, Uemura T, Kakugawa Y, et al. Down-regulation of sialidase NEU4 may contribute to invasive properties of human colon cancers. Cancer Sci. 2007;98:299–307.
Monti E, Bassi MT, Bresciani R, Civini S, Croci GL, Papini N, et al. Molecular cloning and characterization of NEU4, the fourth member of the human sialidase gene family. Genomics. 2004;83:445–53.
Harr JC, Gonzalez-Sandoval A, Gasser SM. Histones and histone modifications in perinuclear chromatin anchoring: from yeast to man. EMBO Rep. 2016;17:139–55.
Sun TY, Xie HJ, Li Z, Kong LF, Gou XN, Li DJ, et al. miR-34a regulates HDAC1 expression to affect the proliferation and apoptosis of hepatocellular carcinoma. Am J Transl Res. 2017;9:103–14.
Quint K, Agaimy A, Di Fazio P, Montalbano R, Steindorf C, Jung R, et al. Clinical significance of histone deacetylases 1, 2, 3, and 7: HDAC2 is an independent predictor of survival in HCC. Virchows Arch. 2011;459:129–39.
Chavas LM, Tringali C, Fusi P, Venerando B, Tettamanti G, Kato R, et al. Crystal structure of the human cytosolic sialidase Neu2. Evidence for the dynamic nature of substrate recognition. J Biol Chem. 2005;280:469–75.
Mozzi A, Mazzacuva P, Zampella G, Forcella ME, Fusi PA, Monti E. Molecular insight into substrate recognition by human cytosolic sialidase NEU2. Proteins. 2012;80:1123–32.
Finlay TM, Jayanth P, Amith SR, Gilmour A, Guzzo C, Gee K, et al. Thymoquinone from nutraceutical black cumin oil activates Neu4 sialidase in live macrophage, dendritic, and normal and type I sialidosis human fibroblast cells via GPCR Galphai proteins and matrix metalloproteinase-9. Glycoconj J. 2010;27:329–48.
Zong J, Peng Q, Wang Q, Zhang T, Fan D, Xu X. Human HSP70 and modified HPV16 E7 fusion DNA vaccine induces enhanced specific CD8+ T cell responses and anti-tumor effects. Oncol Rep. 2009;22:953–61.
Zong J, Wang C, Wang Q, Peng Q, Xu Y, Xie X, et al. HSP70 and modified HPV 16 E7 fusion gene without the addition of a signal peptide gene sequence as a candidate therapeutic tumor vaccine. Oncol Rep. 2013;30:3020–6.
Naor D, Sionov RV, Ish-Shalom D. CD44: structure, function, and association with the malignant process. Adv Cancer Res. 1997;71:241–319.
Borland G, Ross JA, Guy K. Forms and functions of CD44. Immunology. 1998;93:139–48.
Katoh S, Zheng Z, Oritani K, Shimozato T, Kincade PW. Glycosylation of CD44 negatively regulates its recognition of hyaluronan. J Exp Med. 1995;182:419–29.
Katoh S, Miyagi T, Taniguchi H, Matsubara Y, Kadota J, Tominaga A, et al. Cutting edge: an inducible sialidase regulates the hyaluronic acid binding ability of CD44-bearing human monocytes. J Immunol. 1999;162:5058–61.
Lesley J, English N, Perschl A, Gregoroff J, Hyman R. Variant cell lines selected for alterations in the function of the hyaluronan receptor CD44 show differences in glycosylation. J Exp Med. 1995;182:431–7.
Rochman M, Moll J, Herrlich P, Wallach SB, Nedvetzki S, Sionov RV, et al. The CD44 receptor of lymphoma cells: structure-function relationships and mechanism of activation. Cell Adhes Commun. 2000;7:331–47.
Guvench O. Revealing the mechanisms of protein disorder and N-glycosylation in CD44-hyaluronan binding using molecular simulation. Front Immunol. 2015;6:305.
Skelton TP, Zeng C, Nocks A, Stamenkovic I. Glycosylation provides both stimulatory and inhibitory effects on cell surface and soluble CD44 binding to hyaluronan. J Cell Biol. 1998;140:431–46.
Li H, Wang X, Zhang C, Cheng Y, Yu M, Zhao K, et al. HDAC1-induced epigenetic silencing of ASPP2 promotes cell motility, tumour growth and drug resistance in renal cell carcinoma. Cancer Lett. 2018;432:121–31.
Liu W, Han F, Qu S, Yao Y, Zhao J, Akhtar ML, et al. MARVELD1 depletion leads to dysfunction of motor and cognition via regulating glia-dependent neuronal migration during brain development. Cell Death Dis. 2018;9:999.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31771627).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, X., Dou, P., Akhtar, M.L. et al. NEU4 inhibits motility of HCC cells by cleaving sialic acids on CD44. Oncogene 40, 5427–5440 (2021). https://doi.org/10.1038/s41388-021-01955-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-01955-7
This article is cited by
-
The role of N-glycosylation modification in the pathogenesis of liver cancer
Cell Death & Disease (2023)
-
Chromatin-associated OGT promotes the malignant progression of hepatocellular carcinoma by activating ZNF263
Oncogene (2023)
-
Protein glycosylation alterations in hepatocellular carcinoma: function and clinical implications
Oncogene (2023)
-
Neuraminidase 1 promotes renal fibrosis development in male mice
Nature Communications (2023)
-
Sialylation of cell surface glycoconjugates modulates cytosolic galectin-mediated responses upon organelle damage
Glycoconjugate Journal (2023)