Abstract
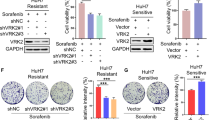
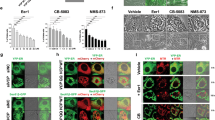
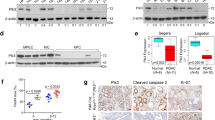
As a key cell cycle regulator, polo-like kinase 1 (Plk1) has been recognized as a crucial factor involved in the progression of pancreatic cancer (PC). However, its regulatory mechanism is poorly understood. Here, we present evidence that Plk1 is a novel substrate of vaccinia-related kinase 2 (VRK2), a serine-threonine kinase that is highly expressed and predicts poor prognosis in PC. VRK2 phosphorylates Plk1 at threonine 210 and protects it from ubiquitin-dependent proteasomal degradation. We showed that mechanistically complement factor H-related protein (CFHR), as a major E3 ligase, promotes Plk1 degradation by ubiquitinating it at lysine 209. Phosphorylation of Plk1 at threonine 210 by VRK2 interferes with the interaction of Chfr with Plk1 and antagonizes Plk1 ubiquitination, thereby stabilizing the Plk1 protein. Taken together, our data reveal a mechanism of Plk1 overexpression in PC and provide evidence for targeting VRK2 as a potential therapeutic strategy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694–705.
Yabar CS, Winter JM. Pancreatic cancer: a review. Gastroenterol Clin North Am. 2016;45:429–45.
McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846–61.
Lin QJ, Yang F, Jin C, Fu DL. Current status and progress of pancreatic cancer in China. World J Gastroenterol. 2015;21:7988–8003.
Grimont A, Pinho AV, Cowley MJ, Augereau C, Mawson A, Giry-Laterrière M, et al. SOX9 regulates ERBB signalling in pancreatic cancer development. Gut. 2015;64:1790–9.
Berry LD, Golsteyn RM, Lane HA, Mundt KE, Arnaud L, Nigg EA. The family of polo-like kinases. Progr Cell Cycle Res. 1996;2:107–14.
Archambault V, Glover DM. Polo-like kinases: conservation and divergence in their functions and regulation. Nat Rev Mol Cell Biol. 2009;10:265–75.
Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer. 2010;10:825–41.
Liu Z, Sun Q, Wang XPLK1. a potential target for cancer therapy. Transl Oncol. 2017;10:22–32.
de Carcer G, Venkateswaran SV, Salgueiro L, El Bakkali A, Somogyi K, Rowald K, et al. Plk1 overexpression induces chromosomal instability and suppresses tumor development. Nat Commun. 2018;9:3012.
Shin CH, Lee H, Kim HR, Choi KH, Joung JG, Kim HH. Regulation of PLK1 through competition between hnRNPK, miR-149-3p and miR-193b-5p. Cell Death Differ. 2017;24:1861–71.
Xin X, Lin F, Wang Q, Yin L, Mahato RI. ROS-responsive polymeric micelles for triggered simultaneous delivery of PLK1 inhibitor/miR-34a and effective synergistic therapy in pancreatic cancer. ACS Appl Mater Interfaces. 2019;11:14647–59.
Li J, Wang R, Schweickert PG, Karki A, Yang Y, Kong Y, et al. Plk1 inhibition enhances the efficacy of gemcitabine in human pancreatic cancer. Cell Cycle. 2016;15:711–9.
Song B, Liu XS, Rice SJ, Kuang S, Elzey BD, Konieczny SF, et al. Plk1 phosphorylation of Orc2 and Hbo1 contributes to gemcitabine resistance in pancreatic cancer. Mol Cancer Therap. 2012;12:58–68.
Nezu J, Oku A, Jones MH, Shimane M. Identification of two novel human putative serine/threonine kinases, VRK1 and VRK2, with structural similarity to vaccinia virus B1R kinase. Genomics. 1997;45:327–31.
Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–34.
Nichols RJ, Traktman P. Characterization of three paralogous members of the Mammalian vaccinia related kinase family. J Biol Chem. 2004;279:7934–46.
Klerkx EP, Lazo PA, Askjaer P. Emerging biological functions of the vaccinia-related kinase (VRK) family. 2009.
Tesli M, Wirgenes KV, Hughes T, Bettella F, Athanasiu L, Hoseth ES, et al. VRK2 gene expression in schizophrenia, bipolar disorder and healthy controls. Br J Psychiatry. 2016;209:114–20.
Sanz-García M, López-Sánchez I, Lazo PA. Proteomics identification of nuclear Ran GTPase as an inhibitor of human VRK1 and VRK2 (vaccinia-related kinase) activities. Mol Cell Proteom. 2008;7:2199–214.
Vázquez-Cedeira M, Lazo PA. Human VRK2 (vaccinia-related kinase 2) modulates tumor cell invasion by hyperactivation of NFAT1 and expression of cyclooxygenase-2. J Biol Chem. 2012;287:42739–50.
Kim S, Lee D, Lee J, Song H, Kim H-J, Kim K-T. Vaccinia-related kinase 2 controls the stability of the eukaryotic chaperonin TRiC/CCT by inhibiting the deubiquitinating enzyme USP25. Mol Cell Biol. 2015;35:1754–62.
Fernández IF, Blanco S, Lozano J, Lazo PA. VRK2 inhibits mitogen-activated protein kinase signaling and inversely correlates with ErbB2 in human breast cancer. Mol Cell Biol. 2010;30:4687–97.
Blanco S, Klimcakova L, Vega FM, Lazo PA. The subcellular localization of vaccinia‐related kinase‐2 (VRK2) isoforms determines their different effect on p53 stability in tumour cell lines. FEBS J. 2006;273:2487–504.
Kim S, Park D-Y, Lee D, Kim W, Jeong Y-H, Lee J, et al. Vaccinia-related kinase 2 mediates accumulation of polyglutamine aggregates via negative regulation of the chaperonin TRiC. Mol Cell Biol. 2014;34:643–52.
Shin S-B, Jang H-R, Xu R, Won J-Y, Yim H. Active PLK1-driven metastasis is amplified by TGF-β signaling that forms a positive feedback loop in non-small cell lung cancer. Oncogene. 2020;39:767–85.
Kang D, Chen J, Wong J, Fang G. The checkpoint protein Chfr is a ligase that ubiquitinates Plk1 and inhibits Cdc2 at the G2 to M transition. J Cell Biol. 2002;156:249–60.
Chen B, Zhu A, Tian L, Xin Y, Liu X, Peng Y, et al. miR23a suppresses pancreatic cancer cell progression by inhibiting PLK1 expression. Mol Med Rep. 2018;18:105–12.
Mahajan UM, Teller S, Sendler M, Palankar R, van den Brandt C, Schwaiger T, et al. Tumour-specific delivery of siRNA-coupled superparamagnetic iron oxide nanoparticles, targeted against PLK1, stops progression of pancreatic cancer. Gut. 2016;65:1838–49.
Monsalve DM, Merced T, Fernandez IF, Blanco S, Vazquez-Cedeira M, Lazo PA. Human VRK2 modulates apoptosis by interaction with Bcl-xL and regulation of BAX gene expression. Cell Death Dis. 2013;4:e513.
Xiao D, Yue M, Su H, Ren P, Jiang J, Li F, et al. Polo-like kinase-1 regulates myc stabilization and activates a feedforward circuit promoting tumor cell survival. Mol Cell. 2016;64:493–506.
Mai J, Zhong ZY, Guo GF, Chen XX, Xiang YQ, Li X, et al. Polo-Like Kinase 1 phosphorylates and stabilizes KLF4 to promote tumorigenesis in nasopharyngeal carcinoma. Theranostics. 2019;9:3541–54.
Wen D, Wu J, Wang L, Fu Z. SUMOylation promotes nuclear import and stabilization of polo-like kinase 1 to support its mitotic function. Cell Rep. 2017;21:2147–59.
Eckerdt F, Yuan J, Saxena K, Martin B, Kappel S, Lindenau C, et al. Polo-like kinase 1-mediated phosphorylation stabilizes Pin1 by inhibiting its ubiquitination in human cells. J Biol Chem. 2005;280:36575–83.
Jeong YH, Choi JH, Lee D, Kim S, Kim KT. Vaccinia-related kinase 2 modulates role of dysbindin by regulating protein stability. J Neurochem. 2018;147:609–25.
Blanco S, Klimcakova L, Vega FM, Lazo PA. The subcellular localization of vaccinia-related kinase-2 (VRK2) isoforms determines their different effect on p53 stability in tumour cell lines. FEBS J. 2006;273:2487–504.
Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science. 2008;320:1655–8.
Qian YW, Erikson E, Maller JL. Mitotic effects of a constitutively active mutant of the Xenopus polo-like kinase Plx1. Mol Cell Biol. 1999;19:8625–32.
Kang D, Chen J, Wong J, Fang G. The checkpoint protein Chfr is a ligase that ubiquitinates Plk1 and inhibits Cdc2 at the G2 to M transition. J Cell Biol. 2002;156:249–59.
Kim JS, Park YY, Park SY, Cho H, Kang D, Cho H. The auto-ubiquitylation of E3 ubiquitin-protein ligase Chfr at G2 phase is required for accumulation of polo-like kinase 1 and mitotic entry in mammalian cells. J Biol Chem. 2011;286:30615–23.
Acknowledgements
This study was supported by the Scientific research and cultivation project of talents in the First Affiliated Hospital of Nanchang University (YFYPY202015).
Author information
Authors and Affiliations
Contributions
SSC and BX conceived, designed and supervised the execution of the entire project. HQZ, QL and YLZ initiated the project, designed experiments, and carried out major western blot, bioinformatics, cellular and animal studies. HP and JZ helped to establish IHC staining and pathological analyses. LYG and ZJ were responsible for mass spectrometry analysis. ZXZ analyzed and interpreted the data. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
COMPETING INTERESTS
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhu, H., Li, Q., Zhao, Y. et al. Vaccinia-related kinase 2 drives pancreatic cancer progression by protecting Plk1 from Chfr-mediated degradation. Oncogene 40, 4663–4674 (2021). https://doi.org/10.1038/s41388-021-01893-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-01893-4
This article is cited by
-
Elevated FBXL6 expression in hepatocytes activates VRK2-transketolase-ROS-mTOR-mediated immune evasion and liver cancer metastasis in mice
Experimental & Molecular Medicine (2023)