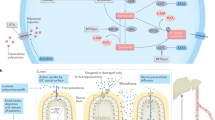
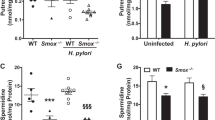
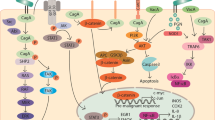
Abstract
Advancements in our understanding of polyamine molecular and cellular functions have led to increased interest in targeting polyamine metabolism for anticancer therapeutic benefits. The polyamines putrescine, spermidine, and spermine are polycationic alkylamines commonly found in all living cells and are essential for cellular growth and survival. This review summarizes the existing research on polyamine metabolism and function, specifically the role of polyamines in gastric immune cell and epithelial cell function. Polyamines have been implicated in a multitude of cancers, but in this review, we focus on the role of polyamine dysregulation in the context of Helicobacter pylori-induced gastritis and subsequent progression to gastric cancer. Due to the emerging implication of polyamines in cancer development, there is an increasing number of promising clinical trials using agents to target the polyamine metabolic pathway for potential chemoprevention and anticancer therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26–38.
Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–First American Cancer Society Award lecture on cancer epidemiology and prevention. Cancer Res. 1992;52:6735–40.
Correa P, Piazuelo BM, Wilson KT. Pathology of gastric intestinal metaplasia: clinical implications. Am J Gastroenterol. 2010;105:493–8.
Correa P, Shiao Y. Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res. 1994;54:1941s–3s.
Piazuelo MB, Bravo LE, Mera RM, Camargo MC, Bravo JC, Delgado AG, et al. The Colombian chemoprevention trial: 20-year follow-up of a cohort of patients with gastric precancerous lesions. Gastroenterology. 2021;160:1106–17.
Ge S, Xia X, Ding C, Zhen B, Zhou Q, Feng J, et al. A proteomic landscape of diffuse-type gastric cancer. Nat Commun. 2018;9:1012.
Hooi JKY, Lai WY, Ng WK, Suen MMY, Underwood FE, Tanyingoh D, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420–9.
Wroblewski LE, Peek RM, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23:713–39.
Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N. Engl J Med. 2001;345:784–9.
Romero-Gallo J, Harris EJ, Krishna U, Washington MK, Perez-Perez GI, Peek RM. Effect of Helicobacter pylori eradication on gastric carcinogenesis. Lab Invest. 2008;88:328–36.
Ma J-L, Zhang L, Brown LM, Li J-Y, Shen L, Pan K-F, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012;104:488–92.
You W, Brown LM, Zhang L, Li J, Jin M, Chang Y, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst. 2006;98:974–83.
Mera R, Fontham ETH, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, et al. Long term follow up of patients treated for Helicobacter pylori infection. Gut. 2005;54:1536–40.
Camargo MC, Piazuelo MB, Mera RM, Fontham ETH, Delgado AG, Yepez MC, et al. Effect of smoking on failure of H. pylori therapy and gastric histology in a high gastric cancer risk area of Colombia. Acta Gastroenterol Latinoam. 2007;37:238–45.
Blaser MJ. Ecology of Helicobacter pylori in the human stomach. J Clin Invest. 1997;100:759–62.
Mera RM, Bravo LE, Camargo MC, Bravo JC, Delgado AG, Romero-Gallo J, et al. Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut. 2018;67:1239–46.
Oliveira C, Pinheiro H, Figueiredo J, Seruca R, Carneiro F. Familial gastric cancer: genetic susceptibility, pathology, and implications for management. Lancet Oncol. 2015;16:e60–70.
Kountouras J, Zavos C, Chatzopoulos D. New concepts of molecular biology on gastric carcinogenesis. Hepatogastroenterology 2005;52:1305–12.
Li D, Lo W, Rudloff U. Merging perspectives: genotype-directed molecular therapy for hereditary diffuse gastric cancer (HDGC) and E-cadherin-EGFR crosstalk. Clin Transl Med. 2018;7:7.
Etemadi A, Safiri S, Sepanlou SG, Ikuta K, Bisignano C, Shakeri R, et al. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet. Gastroenterol Hepatol. 2020;5:42–54.
Hardbower DM, Peek RM, Wilson KT. At the bench: Helicobacter pylori, dysregulated host responses, DNA damage, and gastric cancer. J Leukoc Biol. 2014;96:201–12.
Gobert AP, Wilson KT. Human and Helicobacter pylori interactions determine the outcome of gastric diseases. Curr Top Microbiol Immunol. 2017;400:27–52.
Pegg AE, McCann PP. Polyamine metabolism and function. Am J Physiol Cell Physiol. 1982;243:C212–21.
Michael AJ. Polyamines in eukaryotes, bacteria, and archaea. J Biol Chem. 2016;291:14896–903.
Pegg AE. Mammalian polyamine metabolism and function. IUBMB Life 2009;61:880–94.
Wei G, Hobbs CA, Defeo K, Hayes CS, Gilmour SK. Polyamine-mediated regulation of protein acetylation in murine skin and tumors. Mol Carcinog. 2007;46:611–7.
Munder M. Arginase: an emerging key player in the mammalian immune system. Br J Pharmacol. 2009;158:638–51.
Jenkinson CP, Grody WW, Cederbaum SD. Comparative properties of arginases. Comp Biochem Physiol B Biochem Mol Biol. 1996;114:107–32.
Pegg AE. Regulation of ornithine decarboxylase. J Biol Chem. 2006;281:14529–32.
Asim M, Chaturvedi R, Hoge S, Lewis ND, Singh K, Barry DP, et al. Helicobacter pylori induces ERK-dependent formation of a phospho-c-Fos·c-Jun activator protein-1 complex that causes apoptosis in macrophages. J Biol Chem. 2010;285:20343–57.
Wang X, Ying W, Dunlap KA, Lin G, Satterfield MC, Burghardt RC, et al. Arginine decarboxylase and agmatinase: an alternative pathway for de novo biosynthesis of polyamines for development of mammalian conceptuses. Biol Reprod. 2014;90:84.
LoGiudice N, Le L, Abuan I, Leizorek Y, Roberts SC. Alpha-difluoromethylornithine, an irreversible inhibitor of polyamine biosynthesis, as a therapeutic strategy against hyperproliferative and infectious diseases. Med Sci. (Basel) 2018;6:12.
Matsui I, Wiegand L, Pegg AE. Properties of spermidine N-acetyltransferase from livers of rats treated with carbon tetrachloride and its role in the conversion of spermidine into putrescine. J Biol Chem. 1981;256:2454–9.
Vujcic S, Liang P, Diegelman P, Kramer DL, Porter CW. Genomic identification and biochemical characterization of the mammalian polyamine oxidase involved in polyamine back-conversion. Biochem J. 2003;370:19–28.
Kramer DL, Diegelman P, Jell J, Vujcic S, Merali S, Porter CW. Polyamine acetylation modulates polyamine metabolic flux, a prelude to broader metabolic consequences. J Biol Chem. 2008;283:4241–51.
Wang Y, Murray-Stewart T, Devereux W, Hacker A, Frydman B, Woster PM, et al. Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem Biophys Res Commun. 2003;304:605–11.
Gobert AP, Al-Greene NT, Singh K, Coburn LA, Sierra JC, Verriere TG, et al. Distinct immunomodulatory effects of spermine oxidase in colitis induced by epithelial injury or infection. Front Immunol. 2018;9:1242.
Vakal S, Jalkanen S, Dahlström KM, Salminen TA. Human copper-containing amine oxidases in drug design and development. Molecules 2020;25:1293.
Park MH, Cooper HL, Folk JE. Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc Natl Acad Sci USA 1981;78:2869–73.
Park MH, Lee YB, Joe YA. Hypusine is essential for eukaryotic cell proliferation. Biol Signals 1997;6:115–23.
Chen K, Liu A. Biochemistry and function of hypusine formation on eukaryotic initiation factor 5A. Biol Signals 1997;6:105–9.
Murphey RJ, Gerner EW. Hypusine formation in protein by a two-step process in cell lysates. J Biol Chem. 1987;262:15033–6.
Abbruzzese A, Park MH, Folk JE. Deoxyhypusine hydroxylase from rat testis. Partial purification and characterization. J Biol Chem. 1986;261:3085–9.
Nakanishi S, Cleveland JL. Targeting the polyamine-hypusine circuit for the prevention and treatment of cancer. Amino Acids 2016;48:2353–62.
Jenkins ZA, Hååg PG, Johansson HE. Human eIF5A2 on chromosome 3q25-q27 is a phylogenetically conserved vertebrate variant of eukaryotic translation initiation factor 5A with tissue-specific expression. Genomics 2001;71:101–9.
Maier B, Ogihara T, Trace AP, Tersey SA, Robbins RD, Chakrabarti SK, et al. The unique hypusine modification of eIF5A promotes islet β cell inflammation and dysfunction in mice. J Clin Invest. 2010;120:2156–70.
Xu A, Jao DL-E, Chen KY. Identification of mRNA that binds to eukaryotic initiation factor 5A by affinity co-purification and differential display. Biochem J. 2004;384:585–90.
Xu A, Chen KY. Hypusine is required for a sequence-specific interaction of eukaryotic initiation factor 5A with postsystematic evolution of ligands by exponential enrichment RNA. J Biol Chem. 2001;276:2555–61.
Aksu M, Trakhanov S, Görlich D. Structure of the exportin Xpo4 in complex with RanGTP and the hypusine-containing translation factor eIF5A. Nat Commun. 2016;7:11952.
Park MH, Wolff EC. Hypusine, a polyamine-derived amino acid critical for eukaryotic translation. J Biol Chem. 2018;293:18710–8.
Pelechano V, Alepuz P. eIF5A facilitates translation termination globally and promotes the elongation of many non-polyproline-specific tripeptide sequences. Nucleic Acids Res. 2017;45:7326–38.
Schuller AP, Wu CC-C, Dever TE, Buskirk AR, Green R. eIF5A functions globally in translation elongation and termination. Mol Cell 2017;66:194–205.
Gutierrez E, Shin B-S, Woolstenhulme CJ, Kim J-R, Saini P, Buskirk AR, et al. eIF5A promotes translation of polyproline motifs. Mol Cell 2013;51:35–45.
Cano VSP, Jeon GA, Johansson HE, Henderson CA, Park J-H, Valentini SR, et al. Mutational analysis of human eIF5A-1: identification of amino acid residues critical for hypusine modification and eIF5A activity. FEBS J. 2008;275:44–58.
Schnier J, Schwelberger HG, Smit-McBride Z, Kang HA, Hershey JW. Translation initiation factor 5A and its hypusine modification are essential for cell viability in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:3105–14.
Sasaki K, Abid MR, Miyazaki M. Deoxyhypusine synthase gene is essential for cell viability in the yeast Saccharomyces cerevisiae. FEBS Lett 1996;384:151–4.
Park J-H, Aravind L, Wolff EC, Kaevel J, Kim YS, Park MH. Molecular cloning, expression, and structural prediction of deoxyhypusine hydroxylase: a HEAT-repeat-containing metalloenzyme. Proc Natl Acad Sci USA 2006;103:51–6.
Nishimura K, Lee SB, Park JH, Park MH. Essential role of eIF5A-1 and deoxyhypusine synthase in mouse embryonic development. Amino Acids 2012;42:703–10.
Henning S, Pällmann N, Miller KK, Hermans-Borgmeyer I, Venz S, Sendoel A, et al. A novel mouse model for inhibition of DOHH-mediated hypusine modification reveals a crucial function in embryonic development, proliferation and oncogenic transformation. Dis Model Mech. 2014;7:963–76.
Pällmann N, Braig M, Sievert H, Preukschas M, Hermans-Borgmeyer I, Schweizer M, et al. Biological relevance and therapeutic potential of the hypusine modification system. J Biol Chem. 2015;290:18343–60.
Coni S, Serrao SM, Yurtsever ZN, Di Magno L, Bordone R, Bertani C, et al. Blockade of EIF5A hypusination limits colorectal cancer growth by inhibiting MYC elongation. Cell Death Dis. 2020;11:1045.
Qiu S, Liu J, Xing F. Antizyme inhibitor 1: a potential carcinogenic molecule. Cancer Sci. 2017;108:163–9.
Patchett SE, Alstead EM, Butruk L, Przytulski K, Farthing MJ. Ornithine decarboxylase as a marker for premalignancy in the stomach. Gut. 1995;37:13–6.
Miao X-P, Li J-S, Li H-Y, Zeng S-P, Zhao Y, Zeng J-Z. Expression of ornithine decarboxylase in precancerous and cancerous gastric lesions. World J Gastroenterol. 2007;13:2867–71.
Chaturvedi R, Asim M, Hoge S, Lewis ND, Singh K, Barry DP, et al. Polyamines impair immunity to Helicobacter pylori by inhibiting L-arginine uptake required for nitric oxide production. Gastroenterology 2010;139:1686–98.
Hardbower DM, Asim M, Luis PB, Singh K, Barry DP, Yang C, et al. Ornithine decarboxylase regulates M1 macrophage activation and mucosal inflammation via histone modifications. Proc Natl Acad Sci USA 2017;114:E751–60.
Elitsur Y, Majumdar AP, Tureaud J, Dosescu J, Neace C, Velusamy L, et al. Tyrosine kinase and ornithine decarboxylase activation in children with Helicobacter pylori gastritis. Life Sci. 1999;65:1373–80.
Konturek PC, Rembiasz K, Konturek SJ, Stachura J, Bielanski W, Galuschka K, et al. Gene expression of ornithine decarboxylase, cyclooxygenase-2, and gastrin in atrophic gastric mucosa infected with Helicobacter pylori before and after eradication therapy. Dig Dis Sci. 2003;48:36–46.
Hirasawa R, Tatsuta M, Iishi H, Yano H, Baba M, Uedo N, et al. Increase in apoptosis and decrease in ornithine decarboxylase activity of the gastric mucosa in patients with atrophic gastritis and gastric ulcer after successful eradication of Helicobacter pylori. Am J Gastroenterol. 1999;94:2398–402.
Takashima T, Fujiwara Y, Watanabe T, Tominaga K, Oshitani N, Higuchi K, et al. High molecular protein of Helicobacter pylori responsible for inhibition of ornithine decarboxylase activity of human gastric cultured cells. Aliment Pharm Ther. 2002;16:167–73.
Xu X, Liu Z, Fang M, Yu H, Liang X, Li X, et al. Helicobacter pylori CagA induces ornithine decarboxylase upregulation via Src/MEK/ERK/c-Myc pathway: implication for progression of gastric diseases. Exp Biol Med (Maywood) 2012;237:435–41.
Gobert AP, Cheng Y, Wang J-Y, Boucher J-L, Iyer RK, Cederbaum SD, et al. Helicobacter pylori induces macrophage apoptosis by activation of arginase II. J Immunol. 2002;168:4692–700.
Cheng Y, Chaturvedi R, Asim M, Bussière FI, Scholz A, Xu H, et al. Helicobacter pylori-induced macrophage apoptosis requires activation of ornithine decarboxylase by c-Myc. J Biol Chem. 2005;280:22492–6.
Chaturvedi R, de Sablet T, Asim M, Piazuelo MB, Barry DP, Verriere TG, et al. Increased Helicobacter pylori-associated gastric cancer risk in the Andean region of Colombia is mediated by spermine oxidase. Oncogene. 2015;34:3429–40.
Murray-Stewart T, Sierra JC, Piazuelo MB, Mera RM, Chaturvedi R, Bravo LE, et al. Epigenetic silencing of miR-124 prevents spermine oxidase regulation: implications for Helicobacter pylori-induced gastric cancer. Oncogene. 2016;35:5480–8.
Sierra JC, Piazuelo MB, Luis PB, Barry DP, Allaman MM, Asim M, et al. Spermine oxidase mediates Helicobacter pylori-induced gastric inflammation, DNA damage, and carcinogenic signaling. Oncogene. 2020;39:4465–74.
Chaturvedi R, Cheng Y, Asim M, Bussière FI, Xu H, Gobert AP, et al. Induction of polyamine oxidase 1 by Helicobacter pylori causes macrophage apoptosis by hydrogen peroxide release and mitochondrial membrane depolarization. J Biol Chem. 2004;279:40161–73.
Chaturvedi R, Asim M, Barry DP, Frye JW, Casero RA, Wilson KT. Spermine oxidase is a regulator of macrophage host response to Helicobacter pylori: enhancement of antimicrobial nitric oxide generation by depletion of spermine. Amino Acids 2014;46:531–42.
Xu F, Xu Y, Xiong J-H, Zhang J-H, Wu J, Luo J, et al. AOC1 contributes to tumor progression by promoting the AKT and EMT pathways in gastric cancer. Cancer Manag Res. 2020;12:1789–98.
Jung MH, Kim SC, Jeon GA, Kim SH, Kim Y, Choi KS, et al. Identification of differentially expressed genes in normal and tumor human gastric tissue. Genomics 2000;69:281–6.
Okugawa Y, Toiyama Y, Shigeyasu K, Yamamoto A, Shigemori T, Yin C, et al. Enhanced AZIN1 RNA editing and overexpression of its regulatory enzyme ADAR1 are important prognostic biomarkers in gastric cancer. J Transl Med. 2018;16:366.
Lundell L, Rosengren E. Polyamine levels in human gastric carcinoma. Scand J Gastroenterol. 1986;21:829–32.
Linsalata M, Russo F, Notarnicola M, Berloco P, Di Leo A. Polyamine profile in human gastric mucosa infected by Helicobacter pylori. Ital J Gastroenterol Hepatol. 1998;30:484–9.
Wilson KT, Crabtree JE. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology 2007;133:288–308.
Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69.
Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime Rep. 2014;6:13.
Anderson CF, Mosser DM. A novel phenotype for an activated macrophage: the type 2 activated macrophage. J Leukoc Biol. 2002;72:101–6.
Gobert AP, Finley JL, Latour YL, Asim M, Smith TM, Verriere TG, et al. Hypusination orchestrates the antimicrobial response of macrophages. Cell Rep. 2020;33:108510.
Bussière FI, Chaturvedi R, Cheng Y, Gobert AP, Asim M, Blumberg DR, et al. Spermine causes loss of innate immune response to Helicobacter pylori by inhibition of inducible nitric-oxide synthase translation. J Biol Chem. 2005;280:2409–12.
Xu C, Yan Y, Yang Y, Liu B, Xin J, Chen S, et al. Downregulation of ornithine decarboxylase by pcDNA-ODCr inhibits gastric cancer cell growth in vitro. Mol Biol Rep. 2011;38:949–55.
Lewis ND, Asim M, Barry DP, de Sablet T, Singh K, Piazuelo MB, et al. Immune evasion by Helicobacter pylori is mediated by induction of macrophage arginase II. J Immunol. 2011;186:3632–41.
Gamble LD, Purgato S, Murray J, Xiao L, Yu DMT, Hanssen KM, et al. Inhibition of polyamine synthesis and uptake reduces tumor progression and prolongs survival in mouse models of neuroblastoma. Sci Transl Med. 2019;11:eaau1099.
Devens BH, Weeks RS, Burns MR, Carlson CL, Brawer MK. Polyamine depletion therapy in prostate cancer. Prostate Cancer Prostatic Dis. 2000;3:275–9.
Milovic V, Turchanowa L. Polyamines and colon cancer. Biochem Soc Trans. 2003;31:381–3.
Saydjari R, Alexander RW, Upp JR, Poston GJ, Barranco SC, Townsend CM, et al. The effect of tumor burden on ornithine decarboxylase activity in mice. Cancer Invest. 1991;9:415–9.
Xu H, Chaturvedi R, Cheng Y, Bussiere FI, Asim M, Yao MD, et al. Spermine oxidation induced by Helicobacter pylori results in apoptosis and DNA Damage: implications for gastric carcinogenesis. Cancer Res. 2004;64:8521–5.
Wang JY, Johnson LR. Luminal polyamines stimulate repair of gastric mucosal stress ulcers. Am J Physiol. 1990;259:G584–592.
Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517.
Hardbower DM, Singh K, Asim M, Verriere TG, Olivares-Villagómez D, Barry DP, et al. EGFR regulates macrophage activation and function in bacterial infection. J Clin Invest. 2016;126:3296–312.
Barry DP, Asim M, Leiman DA, de Sablet T, Singh K, Casero RA, et al. Difluoromethylornithine is a novel inhibitor of Helicobacter pylori growth, CagA translocation, and interleukin-8 induction. PLoS ONE 2011;6:e17510.
Sierra JC, Suarez G, Piazuelo MB, Luis PB, Baker DR, Romero-Gallo J, et al. α-Difluoromethylornithine reduces gastric carcinogenesis by causing mutations in Helicobacter pylori cagY. Proc Natl Acad Sci USA 2019;116:5077–85.
Ehrnström RA, Veress B, Arvidsson S, Sternby NH, Andersson T, Lindström CG. Dietary supplementation of carbonate promotes spontaneous tumorigenesis in a rat gastric stump model. Scand J Gastroenterol. 2006;41:12–20.
Iishi H, Tatsuta M, Baba M, Yano H, Sakai N, Uehara H, et al. Ornithine decarboxylase inhibitor lessens the rat gastric carcinogenesis enhancement caused by tyrosine methyl ester. Int J Cancer. 1997;73:113–6.
Tatsuta M, Iishi H, Baba M, Yano H, Uehara H, Nakaizumi A. Ornithine decarboxylase inhibitor attenuates NaCl enhancement of gastric carcinogenesis induced by N-methyl-N’-nitro-N-nitrosoguanidine. Carcinogenesis 1995;16:2107–10.
Prakash NJ, Schechter PJ, Grove J, Koch-Weser J. Effect of alpha-difluoromethylornithine, an enzyme-activated irreversible inhibitor of ornithine decarboxylase, on L1210 leukemia in mice. Cancer Res. 1978;38:3059–62.
Mamont PS, Duchesne M-C, Grove J, Bey P. Anti-proliferative properties of DL-α-difluoromethyl ornithine in cultured cells. A consequence of the irreversible inhibition of ornithine decarboxylase. Biochem Biophys Res Commun. 1978;81:58–66.
Horn Y, Schechter PJ, Marton LJ. Phase I-II clinical trial with alpha-difluoromethylornithine-an inhibitor of polyamine biosynthesis. Eur J Cancer Clin Oncol. 1987;23:1103–7.
Nass MM. Analysis of methylglyoxal bis(guanylhydrazone)-induced alterations of hamster tumor mitochondria by correlated studies of selective rhodamine binding, ultrastructural damage, DNA replication, and reversibility. Cancer Res. 1984;44:2677–88.
Pless M, Belhadj K, Menssen HD, Kern W, Coiffier B, Wolf J, et al. Clinical efficacy, tolerability, and safety of SAM486A, a novel polyamine biosynthesis inhibitor, in patients with relapsed or refractory non-Hodgkin’s lymphoma: results from a phase II multicenter study. Clin Cancer Res. 2004;10:1299–305.
Zuylen L, van, Bridgewater J, Sparreboom A, Eskens FALM, Bruijn P de, Sklenar I. et al. Phase I and pharmacokinetic study of the polyamine synthesis inhibitor SAM486A in combination with 5-fluorouracil/ leucovorin in metastatic colorectal cancer. Clin Cancer Res. 2004;10:1949–55.
Millward MJ, Joshua A, Kefford R, Aamdal S, Thomson D, Hersey P, et al. Multi-centre phase II trial of the polyamine synthesis inhibitor SAM486A (CGP48664) in patients with metastatic melanoma. Invest N Drugs. 2005;23:253–6.
Sholler GLS, Ferguson W, Bergendahl G, Bond JP, Neville K, Eslin D, et al. Maintenance DFMO increases survival in high-risk neuroblastoma. Sci Rep. 2018;8:14445.
Meyskens FL, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Philos Pa) 2008;1:32–8.
Acknowledgements
This work was funded by NIH grants R01CA190612 (K.T.W.), P01CA116087 (K.T.W.), P01CA028842 (K.T.W.), and R21AI142042 (K.T.W.); Veterans Affairs Merit Review grants I01BX001453 and I01CX002171 (K.T.W.); Department of Defense grant W81XWH-18-1-0301 (K.T.W.); Crohn’s & Colitis Foundation Senior Research Award 703003 (K.T.W.); the Thomas F. Frist Sr. Endowment (K.T.W.); and the Vanderbilt Center for Mucosal Inflammation and Cancer (K.T.W.). K.T.W. also receives support from the Vanderbilt Digestive Disease Research Center (NIH grant P30DK058404). K.M.M. was supported by NIH grant T32CA009592.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McNamara, K.M., Gobert, A.P. & Wilson, K.T. The role of polyamines in gastric cancer. Oncogene 40, 4399–4412 (2021). https://doi.org/10.1038/s41388-021-01862-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-01862-x
This article is cited by
-
Spatially resolved multi-omics highlights cell-specific metabolic remodeling and interactions in gastric cancer
Nature Communications (2023)
-
Dietary factors associated with gastric cancer - a review
Translational Medicine Communications (2022)
-
Elevation of spermine remodels immunosuppressive microenvironment through driving the modification of PD-L1 in hepatocellular carcinoma
Cell Communication and Signaling (2022)
-
Polyamines in cancer: integrating organismal metabolism and antitumour immunity
Nature Reviews Cancer (2022)
-
Specific and sensitive GC–MS analysis of hypusine, Nε-(4-amino-2-hydroxybutyl)lysine, a biomarker of hypusinated eukaryotic initiation factor eIF5A, and its application to the bi-ethnic ASOS study
Amino Acids (2022)