Abstract
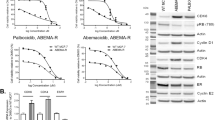
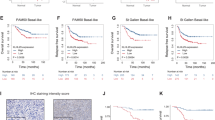
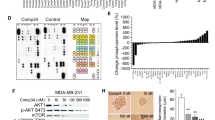
Molecular alterations in the PI3K/AKT pathway occur frequently in hormone receptor-positive breast tumors. Patients with ER-positive, HER2-negative metastatic breast cancer are often treated with CDK4/6 inhibitors such as palbociclib in combination with endocrine therapy. Although this is an effective regimen, most patients ultimately progress. The purpose of this study was identifying synthetic lethality partners that can enhance palbociclib’s antitumor efficacy in the presence of PIK3CA/AKT1 mutations. We utilized a barcoded shRNA library to determine critical targets for survival in isogenic MCF7 cells with PIK3CA/AKT1 mutations. We demonstrated that the efficacy of palbociclib is reduced in the presence of PIK3CA/AKT1 mutations. We also identified that the downregulation of discoidin domain receptor 1 (DDR1) is synthetically lethal with palbociclib. DDR1 knockdown and DDR1 pharmacological inhibitor decreased cell growth and inhibited cell cycle progression in all cell lines, while enhanced the sensitivity of PIK3CA/AKT1 mutant cells to palbociclib. Combined treatment of palbociclib and 7rh further induced cell cycle arrest in PIK3CA/AKT1 mutant cell lines. In vivo, 7rh significantly enhanced palbociclib’s antitumor efficacy. Our data indicates that DDR1 inhibition can augment cell cycle suppressive effect of palbociclib and could be effective strategy for targeted therapy of ER-positive, HER2-negative breast cancers with PI3K pathway activation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Polyak K. Heterogeneity in breast cancer. J Clin Investig. 2011;121:3786–8.
Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–7.
Miller TW, Rexer BN, Garrett JT, Arteaga CL. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res. 2011;13:224
Meric-Bernstam F, Zheng X, Shariati M, Damodaran S, Wathoo C, Brusco L, et al. Survival outcomes by TP53 mutation status in metastatic breast cancer. JCO Precis Oncol. 2018; 2: PO.17.00245.
Shariati M, Meric-Bernstam F. Targeting AKT for cancer therapy. Expert Opin Investig Drugs. 2019;1–12.
Andre F, Ciruelos EM, Rubovszky G, Campone M, Loibl S, Rugo HS. et al. Alpelisib (ALP) 1 fulvestrant (FUL) for advanced breast cancer (ABC): results of the phase III SOLAR-1 trial. Ann Oncol. 2018;29:709.
Hyman DM, Smyth LM, Donoghue MTA, Westin SN, Bedard PL, Dean EJ. et al. AKT inhibition in solid tumors with AKT1 mutations. J Clin Oncol. 2017;35:2251–9.
Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:5.
Toogood PL, Harvey PJ, Repine JT, Sheehan DJ, VanderWel SN, Zhou HR. et al. Discovery of a potent and selective inhibitor of cyclin-dependent kinase 4/6. J Medicinal Chem. 2005;48:2388–406.
Wander SA, Cohen O, Gong XQ, Johnson GN, Buendia-Buendia J, Lloyd M, et al. The genomic landscape of intrinsic and acquired resistance to cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) in patients with hormone receptor-positive (HR+)/HER2-metastatic breast cancer (MBC). Cancer Res. 2020;80:4.
Herrera-Abreu MT, Palafox M, Asghar U, Rivas MA, Cutts RJ, Garcia-Murillas I, et al. Early adaptation and acquired resistance to CDK4/6 inhibition in estrogen receptor-positive breast cancer. Cancer Research. 2016;76:5907.
Jansen VM, Bhola NE, Bauer JA, Formisano L, Lee KM, Hutchinson KE. et al. Kinome-Wide RNA Interference Screen Reveals a Role for PDK1 in Acquired Resistance to CDK4/6 Inhibition in ER-Positive Breast Cancer. Cancer Res. 2017;77:2488–99.
Cortes J, Im SA, Holgado E, Perez-Garcia JM, Schmid P, Chavez-MacGregor M. The next era of treatment for hormone receptor-positive, HER2-negative advanced breast cancer: triplet combination-based endocrine therapies. Cancer Treat Rev. 2017;61:53–60.
Juric D, Ismail-Khan R, Campone M, Estevez Garcia L, Becerra C, Boer R. et al. Abstract P3-14-01: phase Ib/II study of ribociclib and alpelisib and letrozole in ER+, HER2– breast cancer: safety, preliminary efficacy and molecular analysis. Cancer Res. 2016;76:P3–14.
Vora SR, Juric D, Kim N, Mino-Kenudson M, Huynh T, Costa C. et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell. 2014;26:136–49.
Gadiya M, Chakraborty G. Signaling by discoidin domain receptor 1 in cancer metastasis. Cell Adh Migr. 2018;12:315–23.
Belfiore A, Malaguarnera R, Nicolosi ML, Lappano R, Ragusa M, Morrione A. et al. A novel functional crosstalk between DDR1 and the IGF axis and its relevance for breast cancer. Cell Adh Migration. 2018;12:305–14.
Beaver JA, Gustin JP, Yi KH, Rajpurohit A, Thomas M, Gilbert SF. et al. PIK3CA and AKT1 Mutations Have Distinct Effects on Sensitivity to Targeted Pathway Inhibitors in an Isogenic Luminal Breast Cancer Model System. Clin Cancer Res. 2013;19:5413–22.
Palbociclib package insert [PDF on the Internet]:2016 [cited 2020 March 2]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/207103s002lbl.pdf.
Day PJ, Cleasby A, Tickle IJ, O’Reilly M, Coyle JE, Holding FP. et al. Crystal structure of human CDK4 in complex with a D-type cyclin. Proc Natl Acad Sci U.S.A. 2009;106:4166–70.
Moon A, Koh M, Woo Y, Lee D, Valiathan R, Jung HY.et al. Discoidin domain receptor (DDR) 1 is a novel transcriptional target of ZEB1 in breast epithelial cells undergoing H-ras-induced epithelial to mesenchymal transition. Faseb J. 2015;136:E508–E520..
Saby C, Collin G, Sinane M, Buache E, Van Gulick L, Saltel F, et al. DDR1 and MT1-MMP expression levels are determinant for triggering BIK-mediated apoptosis by 3D type I collagen matrix in invasive basal-like breast carcinoma cells. Front Pharmacol. 2019. 10;462.
Assent D, Bourgot I, Hennuy B, Geurts P, Noel A, Foidart JM, et al. A membrane-type-1 matrix metalloproteinase (MT1-MMP) - discoidin domain receptor 1 axis regulates collagen-induced apoptosis in breast cancer cells. Plos ONE. 2015;10:3.
Mata R, Palladino C, Nicolosi ML, Lo Presti AR, Malaguarnera R, Ragusa M. et al. IGF-I induces upregulation of DDR1 collagen receptor in breast cancer cells by suppressing MIR-199a-5p through the PI3K/AKT pathway. Oncotarget. 2016;7:7683–7700.
Gao M, Duan L, Luo J, Zhang L, Lu X, Zhang Y. et al. Discovery and optimization of 3-(2-(Pyrazolo[1,5-a]pyrimidin-6-yl)ethynyl)benzamides as novel selective and orally bioavailable discoidin domain receptor 1 (DDR1) inhibitors. J Med Chem. 2013;56:3281–95.
Anders L, Ke N, Hydbring P, Choi YJ, Widlund HR, Chick JM. et al. A systematic screen for CDK4/6 substrates links FOXM1 phosphorylation to senescence suppression in cancer cells. Cancer Cell. 2011;20:620–34.
Dean JL, Thangavel C, McClendon AK, Reed CA, Knudsen ES. Therapeutic CDK4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene. 2010;29:4018–32.
Franco J, Witkiewicz AK, Knudsen ES. CDK4/6 inhibitors have potent activity in combination with pathway selective therapeutic agents in models of pancreatic cancer. Oncotarget. 2014;5:6512–25.
Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K. et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8:1153–60.
Rossig L, Jadidi AS, Urbich C, Badorff C, Zeiher AM, Dimmeler S. Akt-dependent phosphorylation of p21(Cip1) regulates PCNA binding and proliferation of endothelial cells. Mol Cell Biol. 2001;21:5644–57.
Das S, Ongusaha PP, Yang YS, Park JM, Aaronson SA, Lee SW. Discoidin domain receptor 1 receptor tyrosine kinase induces cyclooxygenase-2 and promotes chemoresistance through nuclear factor-kappa B pathway activation. Cancer Res. 2006;66:8123–30.
Villalba M, Redin E, Exposito F, Pajares MJ, Sainz C, Hervas D. et al. Identification of a novel synthetic lethal vulnerability in non-small cell lung cancer by co-targeting TMPRSS4 and DDR1. Sci Rep. 2019;9:15400.
Ambrogio C, Gomez-Lopez G, Falcone M, Vidal A, Nadal E, Crosetto N. et al. Combined inhibition of DDR1 and Notch signaling is a therapeutic strategy for KRAS-driven lung adenocarcinoma. Nat Med. 2016;22:270–7.
Hortobagyi GN, Stemmer SM, Burris HA, Yap YS, Sonke GS, Paluch-Shimon S. et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N. Engl J Med. 2016;375:1738–48.
Cristofanilli M, Turner NC, Bondarenko I. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:E270.
Cristofanilli M, DeMichele A, Giorgetti C, Turner NC, Slamon DJ, Im SA. et al. Predictors of prolonged benefit from palbociclib plus fulvestrant in women with endocrine-resistant hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer in PALOMA-3. Eur J Cancer. 2018;104:21–31.
O’Leary B, Cutts RJ, Liu Y, Hrebien S, Huang X, Fenwick K. et al. The genetic landscape and clonal evolution of breast cancer resistance to palbociclib plus fulvestrant in the PALOMA-3 trial. Cancer Discov. 2018;8:1390–403.
Gordus A, Krall JA, Beyer EM, Kaushansky A, Wolf-Yadlin A, Sevecka M. et al. Linear combinations of docking affinities explain quantitative differences in RTK signaling. Mol Syst Biol. 2009;5:235.
Vehlow A, Klapproth E, Jin S, Hannen R, Hauswald M, Bartsch JW. et al. Interaction of discoidin domain receptor 1 with a 14-3-3-beclin-1-Akt1 complex modulates glioblastoma therapy sensitivity. Cell Rep. 2019;26:3672–83.
Hinds PW, Weinberg RA. Tumor-suppressor genes. Curr Opin Genet Dev. 1994;4:135–41.
Brennan P, Babbage JW, Burgering BMT, Groner B, Reif K, Cantrell DA. Phosphatidylinositol 3-kinase couples the interleukin-2 receptor to the cell cycle regulator E2F. Immunity. 1997;7:679–89.
Dogruluk T, Tsang YH, Espitia M, Chen F, Chen T, Chong Z. et al. Identification of variant-specific functions of PIK3CA by rapid phenotyping of rare mutations. Cancer Res. 2015;75:5341–54.
Hu J, He X, Baggerly KA, Coombes KR, Hennessy BT, Mills GB. Non-parametric quantification of protein lysate arrays. Bioinformatics. 2007;23:1986–94.
Ju Z, Liu W, Roebuck PL, Siwak DR, Zhang N, Lu Y. et al. Development of a robust classifier for quality control of reverse-phase protein arrays. Bioinformatics. 2015;31:912–8.
Acknowledgements
We thank Visual Art at the University of Texas, MD Anderson Cancer Center for assistance with the illustration in Fig. 2A. We also thank Susanna Brisendine for administrative assistance.
Funding
This work was funded in part by the Nellie B. Connally Breast Cancer Research Endowment, The Center for Clinical and Translational Sciences (CTSA-Informatics) # 1UL1TR003167-01, the MD Anderson Cancer Center Sheikh Khalifa Bin Zayed Al Nahyan Institute for Personalized Cancer, and the Cancer Prevention Institute of Texas Grant RP150535: Precision Oncology Decision Support Core. The MD Anderson Reverse Phase Protein Array Core Facility and Histology, Pathology, and Imaging Core were funded by the Cancer Center Support Grant (CCSG) funded by National Cancer Institute (NCI) # CA-16672.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MS, KWE, XZ, CA, PKS Ng, YQR, FY, AC, and MDP declare no potential conflict of interest. CT is an employee of Epizyme Inc., and performed contract work for Armo Bioscience. TPH reports personal fees and stock ownership from Cullgen Inc. DT reports receiving commercial research grants from Novartis, Polyphor. He also served as a consultant for Pfizer, AstraZeneca, GlaxoSmithKline, Genomic Heath, OncoPep. FMB reports receiving commercial research grants from Aileron Therapeutics, Inc. AstraZeneca, Bayer Healthcare Pharmaceutical, Calithera Biosciences Inc., Curis Inc., CytomX Therapeutics Inc., Daiichi Sankyo Co. Ltd., Debiopharm International, eFFECTOR Therapeutics, Genentech Inc., Guardant Health Inc., Klus Pharma, Millennium Pharmaceuticals Inc., Novartis, Puma Biotechnology Inc., and Taiho Pharmaceutical Co. She also served as a consultant for AbbVie, Aduro BioTech Inc., DebioPharm, eFFECTOR Therapeutics, F. Hoffman-La Roche Ltd., Genentech Inc., IBM Watson, Infinity Pharmaceutical, Jackson Laboratory, Kolon Life Science, OrigiMed, PACT Pharma, Parexel International, Pfizer Inc., Samsung Bioepis, Seattle Genetics Inc., Tyra Biosciences, Xencor, and Zymeworks. In addition, she has served on advisory committees for Immunomedics, Inflection Biosciences, Mersana Therapeutics, Puma Biotechnology Inc., Seattle Genetics, Silverback Therapeutics, Spectrum Pharmaceuticals and Zentalis.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Shariati, M., Evans, K.W., Zheng, X. et al. Combined inhibition of DDR1 and CDK4/6 induces synergistic effects in ER-positive, HER2-negative breast cancer with PIK3CA/AKT1 mutations. Oncogene 40, 4425–4439 (2021). https://doi.org/10.1038/s41388-021-01819-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-01819-0
This article is cited by
-
Predictive, preventive, and personalized medicine in breast cancer: targeting the PI3K pathway
Journal of Translational Medicine (2024)
-
RAGE inhibition blunts insulin-induced oncogenic signals in breast cancer
Breast Cancer Research (2023)
-
A pan-cancer analysis of DDR1 in prognostic signature and tumor immunity, drug resistance
Scientific Reports (2023)