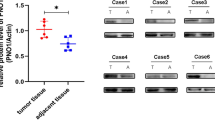
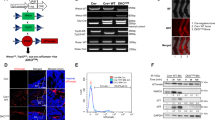
Abstract
Osteosarcoma has a poor prognosis, and the poor understanding of the genetic drivers of osteosarcoma hinders further improvement in therapeutic approaches. Transcription factor forkhead box P1 (FOXP1) is a crucial modulator in skeletal development and aging. Here, we determined the role and regulatory mechanisms of FOXP1 in osteosarcoma. Higher FOXP1 expression correlated with malignancy in both osteosarcoma cell lines and clinical biopsies. FOXP1 overexpression and knockdown in osteosarcoma cell lines revealed that FOXP1 promoted proliferation, tumor sphere formation, migration and invasion, and inhibited anoikis. Mechanistically, FOXP1 acted as a repressor of P21 and RB (retinoblastoma protein) transcription, and directly interacted with the tumor suppressor p53 to inhibit its activity. Extracellular signal-regulated kinase/c-Jun N-terminal kinase (ERK/JNK) signaling and c-JUN/c-FOS transcription factors were found to be upstream activators of FOXP1. Moreover, FOXP1 silencing via lentivirus or adeno-associated virus (AAV)-mediated delivery of shRNA suppressed osteosarcoma development and progression in cell-derived and patient-derived xenograft animal models. Taken together, we demonstrate that FOXP1, which is transactivated by ERK/JNK-c-JUN/c-FOS, drives osteosarcoma development by regulating the p53-P21/RB signaling cascade, suggesting that FOXP1 is a potential target for osteosarcoma therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Gianferante DM, Mirabello L, Savage SA. Germline and somatic genetics of osteosarcoma—connecting aetiology, biology and therapy. Nat Rev Endocrinol. 2017;13:480–91.
Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticanc. 2018;18:39–50.
Lin Y, Jewell BE, Gingold J, Lu L, Zhao R, Wang LL, et al. Osteosarcoma: molecular pathogenesis and iPSC modeling. Trends Mol Med. 2017;23:737–55.
Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer. 2014;14:722–35.
Malkin D. Germline p53 mutations and heritable cancer. Annu Rev Genet. 1994;28:443–65.
Malkin D, Li FP, Strong LC, Fraumeni JJ, Nelson CE, Kim DH, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250:1233–8.
Mirabello L, Yeager M, Mai PL, Gastier-Foster JM, Gorlick R, Khanna C. et al. Germline TP53 variants and susceptibility to osteosarcoma. J Natl Cancer Inst. 2015;107:djv101.
Chen X, Bahrami A, Pappo A, Easton J, Dalton J, Hedlund E, et al. Recurrent somatic structural variations contribute to tumorigenesis in pediatric osteosarcoma. Cell Rep. 2014;7:104–12.
Miller CW, Aslo A, Tsay C, Slamon D, Ishizaki K, Toguchida J, et al. Frequency and structure of p53 rearrangements in human osteosarcoma. Cancer Res. 1990;50:7950.
Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, Roth SI, et al. Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Gene Dev. 2008;22:1662–76.
Berman SD, Calo E, Landman AS, Danielian PS, Miller ES, West JC, et al. Metastatic osteosarcoma induced by inactivation of Rb and p53 in the osteoblast lineage. Proc Natl Acad Sci USA. 2008;105:11851–6.
Armesilla-Diaz A, Elvira G, Silva A. p53 regulates the proliferation, differentiation and spontaneous transformation of mesenchymal stem cells. Exp Cell Res. 2009;315:3598–610.
Lengner CJ, Steinman HA, Gagnon J, Smith TW, Henderson JE, Kream BE, et al. Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling. J Cell Biol. 2006;172:909–21.
Quist T, Jin H, Zhu J, Smith-Fry K, Capecchi MR, Jones KB. The impact of osteoblastic differentiation on osteosarcomagenesis in the mouse. Oncogene. 2015;34:4278–84.
Hansen MF, Koufos A, Gallie BL, Phillips RA, Fodstad O, Brøgger A, et al. Osteosarcoma and retinoblastoma: a shared chromosomal mechanism revealing recessive predisposition. Proc Natl Acad Sci USA. 1985;82:6216.
Wadayama B, Toguchida J, Shimizu T, Ishizaki K, Sasaki MS, Kotoura Y, et al. Mutation spectrum of the retinoblastoma gene in osteosarcomas. Cancer Res. 1994;54:3042.
Fittall MW, Mifsud W, Pillay N, Ye H, Strobl A, Verfaillie A, et al. Recurrent rearrangements of FOS and FOSB define osteoblastoma. Nat Commun. 2018;9:2150.
Wang Z, Liang J, Schellander K, Wagner EF, Grigoriadis AE. c-fos-induced osteosarcoma formation in transgenic mice: cooperativity with c-jun and the role of endogenous c-fos. Cancer Res. 1995;55:6244.
Papachristou DJ, Batistatou A, Sykiotis GP, Varakis I, Papavassiliou AG. Activation of the JNK–AP-1 signal transduction pathway is associated with pathogenesis and progression of human osteosarcomas. Bone. 2003;32:364–71.
Perry JA, Kiezun A, Tonzi P, Van Allen EM, Carter SL, Baca SC, et al. Complementary genomic approaches highlight the PI3K/mTOR pathway as a common vulnerability in osteosarcoma. Proc Natl Acad Sci USA. 2014;111:E5564.
Yang J, Yang D, Sun Y, Sun B, Wang G, Trent JC, et al. Genetic amplification of the vascular endothelial growth factor (VEGF) pathway genes, including VEGFA, in human osteosarcoma. Cancer Am Cancer Soc. 2011;117:4925–38.
Behjati S, Tarpey PS, Haase K, Ye H, Young MD, Alexandrov LB, et al. Recurrent mutation of IGF signalling genes and distinct patterns of genomic rearrangement in osteosarcoma. Nat Commun. 2017;8:15936.
Zhang Y, Li S, Yuan L, Tian Y, Weidenfeld J, Yang J, et al. Foxp1 coordinates cardiomyocyte proliferation through both cell-autonomous and nonautonomous mechanisms. Gene Dev. 2010;24:1746–57.
Li S, Wang Y, Zhang Y, Lu MM, DeMayo FJ, Dekker JD, et al. Foxp1/4 control epithelial cell fate during lung development and regeneration through regulation of anterior gradient 2. Development. 2012;139:2500–9.
Feng X, Wang H, Takata H, Day TJ, Willen J, Hu H. Transcription factor Foxp1 exerts essential cell-intrinsic regulation of the quiescence of naive T cells. Nat Immunol. 2011;12:544–50.
Hu H, Wang B, Borde M, Nardone J, Maika S, Allred L, et al. Foxp1 is an essential transcriptional regulator of B cell development. Nat Immunol. 2006;7:819–26.
Naudin C, Hattabi A, Michelet F, Miri-Nezhad A, Benyoucef A, Pflumio F, et al. PUMILIO/FOXP1 signaling drives expansion of hematopoietic stem/progenitor and leukemia cells. Blood. 2017;129:2493.
Dasen JS, De Camilli A, Wang B, Tucker PW, Jessell TM. Hox repertoires for motor neuron diversity and connectivity gated by a single accessory factor, FoxP1. Cell. 2008;134:304–16.
Usui N, Araujo DJ, Kulkarni A, Co M, Ellegood J, Harper M, et al. Foxp1 regulation of neonatal vocalizations via cortical development. Gene Dev. 2017;31:2039–55.
Leishman E, Howard JM, Garcia GE, Miao Q, Ku AT, Dekker JD, et al. Foxp1 maintains hair follicle stem cell quiescence through regulation of Fgf18. Development. 2013;140:3809–18.
Liu P, Huang S, Ling S, Xu S, Wang F, Zhang W, et al. Foxp1 controls brown/beige adipocyte differentiation and thermogenesis through regulating β3-AR desensitization. Nat Commun. 2019;10:5070.
Gabut M, Samavarchi-Tehrani P, Wang X, Slobodeniuc V, O’Hanlon D, Sung H, et al. An alternative splicing switch regulates embryonic stem cell pluripotency and reprogramming. Cell. 2011;147:132–46.
Li H, Liu P, Xu S, Li Y, Dekker JD, Li B, et al. FOXP1 controls mesenchymal stem cell commitment and senescence during skeletal aging. J Clin Invest. 2017;127:1241–53.
Kim J, Hwang J, Jung JH, Lee H, Lee DY, Kim S. Molecular networks of FOXP family: dual biologic functions, interplay with other molecules and clinical implications in cancer progression. Mol Cancer. 2019;18:180.
Koon HB, Ippolito GC, Banham AH, Tucker PW. FOXP1: a potential therapeutic target in cancer. Expert Opin Ther Tar. 2007;11:955–65.
Flori M, Schmid CA, Sumrall ET, Tzankov A, Law CW, Robinson MD. et al. The hematopoietic oncoprotein FOXP1 promotes tumor cell survival in diffuse large B-cell lymphoma by repressing S1PR2 signaling. Blood. 2016;127:1438–48.
Dekker JD, Park D, Shaffer AL, Kohlhammer H, Deng W, Lee B. et al. Subtype-specific addiction of the activated B-cell subset of diffuse large B-cell lymphoma to FOXP1. Proc Natl Acad Sci USA. 2016;113:E577–86.
Brown PJ, Wong KK, Felce SL, Lyne L, Spearman H, Soilleux EJ, et al. FOXP1 suppresses immune response signatures and MHC class II expression in activated B-cell-like diffuse large B-cell lymphomas. Leukemia. 2016;30:605–16.
Wang X, Sun J, Cui M, Zhao F, Ge C, Chen T. et al. Downregulation of FOXP1 inhibits cell proliferation in hepatocellular carcinoma by inducing G1/S phase cell cycle arrest. Int J Mol Sci. 2016;17:1501.
Fox SB, Brown P, Han C, Ashe S, Leek RD, Harris AL, et al. Expression of the forkhead transcription factor FOXP1 is associated with estrogen receptor α and improved survival in primary human breast carcinomas. Clin Cancer Res. 2004;10:3521–7.
Bates GJ, Fox SB, Han C, Launchbury R, Leek RD, Harris AL, et al. Expression of the forkhead transcription factor FOXP1 is associated with that of estrogen receptor β in primary invasive breast carcinomas. Breast Cancer Res Tr. 2008;111:453–9.
Sheng H, Li X, Xu Y. Knockdown of FOXP1 promotes the development of lung adenocarcinoma. Cancer Biol Ther. 2019;20:537–45.
Takayama K, Horie-Inoue K, Ikeda K, Urano T, Murakami K, Hayashizaki Y, et al. FOXP1 is an androgen-responsive transcription factor that negatively regulates androgen receptor signaling in prostate cancer cells. Biochem Bioph Res Co. 2008;374:388–93.
Mohseny AB, Machado I, Cai Y, Schaefer K, Serra M, Hogendoorn PCW, et al. Functional characterization of osteosarcoma cell lines provides representative models to study the human disease. Lab Invest. 2011;91:1195–205.
Du L, Han X, Tu B, Wang M, Qiao H, Zhang S, et al. CXCR1/Akt signaling activation induced by mesenchymal stem cell-derived IL-8 promotes osteosarcoma cell anoikis resistance and pulmonary metastasis. Cell Death Dis. 2018;9:714.
Huurne MT, Peng T, Yi G, Van Mierlo G, Marks H, Stunnenberg HG. Critical role for P53 in regulating the cell cycle of ground state embryonic stem cells. Stem Cell Rep. 2020;14:175–83.
Jochum W, Passegue E, Wagner EF. AP-1 in mouse development and tumorigenesis. Oncogene. 2001;20:2401–12.
Tao J, Jiang M, Jiang L, Salvo JS, Zeng H, Dawson B, et al. Notch activation as a driver of osteogenic sarcoma. Cancer Cell. 2014;26:390–401.
Deng Q, Li P, Che M, Liu J, Biswas S, Ma G. et al. Activation of hedgehog signaling in mesenchymal stem cells induces cartilage and bone tumor formation via Wnt/β-Catenin. eLife. 2019;8:e50208.
Han Y, Feng H, Sun J, Liang X, Wang Z, Xing W, et al. Lkb1 deletion in periosteal mesenchymal progenitors induces osteogenic tumors through mTORC1 activation. J Clin Invest. 2019;129:1895–909.
Sagaert X, de Paepe P, Libbrecht L, Vanhentenrijk V, Verhoef G, Thomas J, et al. Forkhead box protein P1 expression in mucosa-associated lymphoid tissue lymphomas predicts poor prognosis and transformation to diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:2490–7.
Streubel B, Vinatzer U, Lamprecht A, Raderer M, Chott AT. T (3; 14)(p14. 1; q32) involving IGH and FOXP1 is a novel recurrent chromosomal aberration in MALT lymphoma. Leukemia. 2005;19:652–8.
Mottok A, Jurinovic V, Farinha P, Rosenwald A, Leich E, Ott G. et al. FOXP1 expression is a prognostic biomarker in follicular lymphoma treated with rituximab-containing regimens. Blood. 2018;131:226–35.
Walker MP, Stopford CM, Cederlund M, Fang F, Jahn C, Rabinowitz AD, et al. FOXP1 potentiates Wnt/β-catenin signaling in diffuse large B cell lymphoma. Sci Signal. 2015;8:a12.
van Keimpema M, Grüneberg LJ, Mokry M, van Boxtel R, Koster J, Coffer PJ, et al. FOXP1 directly represses transcription of proapoptotic genes and cooperates with NF-κB to promote survival of human B cells. Blood. 2014;124:3431–40.
Hieronymus H, Iaquinta PJ, Wongvipat J, Gopalan A, Murali R, Mao N. et al. Deletion of 3p13-14 locus spanning FOXP1 to SHQ1 cooperates with PTEN loss in prostate oncogenesis. Nat Commun. 2017;8:1081.
Chen H, Xiao Z, Yu R, Wang Y, Xu R, Zhu X. miR-181d-5p-FOXP1 feedback loop modulates the progression of osteosarcoma. Biochem Bioph Res Co. 2018;503:1434–41.
Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705.
Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–33.
Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–90.
Coventon J. A review of the mechanism of action and clinical applications of sorafenib in advanced osteosarcoma. J Bone Oncol. 2017;8:4–7.
Shu W, Lu MM, Zhang Y, Tucker PW, Zhou D, Morrisey EE. Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development. 2007;134:1991–2000.
Xu S, Liu P, Chen Y, Chen Y, Zhang W, Zhao H, et al. Foxp2 regulates anatomical features that may be relevant for vocal behaviors and bipedal locomotion. Proc Natl Acad Sci USA. 2018;115:8799.
Chokas AL, Trivedi CM, Lu MM, Tucker PW, Li S, Epstein JA, et al. Foxp1/2/4-NuRD interactions regulate gene expression and epithelial injury response in the lung via regulation of interleukin-6. J Biol Chem. 2010;285:13304–13.
Spaeth JM, Hunter CS, Bonatakis L, Guo M, French CA, Slack I, et al. The FOXP1, FOXP2 and FOXP4 transcription factors are required for islet alpha cell proliferation and function in mice. Diabetologia. 2015;58:1836–44.
Zhao H, Zhou W, Yao Z, Wan Y, Cao J, Zhang L, et al. Foxp1/2/4 regulate endochondral ossification as a suppresser complex. Dev Biol. 2015;398:242–54.
Gascoyne DM, Spearman H, Lyne L, Puliyadi R, Perez-Alcantara M, Coulton L, et al. The forkhead transcription factor FOXP2 is required for regulation of p21WAF1/CIP1 in 143B osteosarcoma cell growth arrest. PLoS ONE. 2015;10:e128513.
Yin Z, Ding H, He E, Chen J, Li M. Up-regulation of microRNA-491-5p suppresses cell proliferation and promotes apoptosis by targeting FOXP4 in human osteosarcoma. Cell Prolif. 2017;50:e12308.
van Keimpema M, Gruneberg LJ, Schilder-Tol EJ, Oud ME, Beuling EA, Hensbergen PJ, et al. The small FOXP1 isoform predominantly expressed in activated B cell-like diffuse large B-cell lymphoma and full-length FOXP1 exert similar oncogenic and transcriptional activity in human B cells. Haematologica. 2017;102:573–83.
Xu W, Bian Z, Fan Q, Li G, Tang T. Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett. 2009;281:32–41.
Han X, Yang S, Mo H, Wang M, Zhou F, Li H, et al. Targeting of CXCR1 on osteosarcoma circulating tumor cells and precise treatment via cisplatin nanodelivery. Adv Funct Mater. 2019;29:1902246.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (81802679), the Shanghai Science and Technology Development Fund (18DZ2291200), and China Postdoctoral Science Foundation (2018M632136 and 2019T120348) to HL.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
All animal studies were approved by the Animal Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (HKDL No. 2018–218). All human studies were conducted with the approval of the Independent Ethics Committee of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (No. 2018–80), after receiving written informed consent.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Li, H., Han, X., Yang, S. et al. FOXP1 drives osteosarcoma development by repressing P21 and RB transcription downstream of P53. Oncogene 40, 2785–2802 (2021). https://doi.org/10.1038/s41388-021-01742-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-01742-4
This article is cited by
-
Prognostic and predictive value of super-enhancer-derived signatures for survival and lung metastasis in osteosarcoma
Journal of Translational Medicine (2024)
-
Deacetylation of FOXP1 by HDAC7 potentiates self-renewal of mesenchymal stem cells
Stem Cell Research & Therapy (2023)
-
TRIM69 suppressed the anoikis resistance and metastasis of gastric cancer through ubiquitin‒proteasome-mediated degradation of PRKCD
Oncogene (2023)
-
miR-526b-5p/c-Myc/Foxp1 participates in recurrent spontaneous abortion by regulating the proliferation, migration, and invasion of trophoblasts
Journal of Assisted Reproduction and Genetics (2023)
-
Targeting ferroptosis suppresses osteocyte glucolipotoxicity and alleviates diabetic osteoporosis
Bone Research (2022)