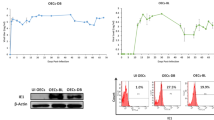
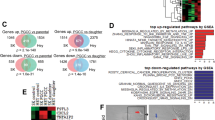
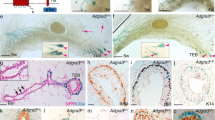
Abstract
A growing body of evidence is recognizing human cytomegalovirus (HCMV) as a potential oncogenic virus. We hereby provide the first experimental in vitro evidence for HCMV as a reprogramming vector, through the induction of dedifferentiation of mature human mammary epithelial cells (HMECs), generation of a polyploid giant cancer cell (PGCC) phenotype characterized by sustained growth of blastomere-like cells, in concordance with the acquisition of embryonic stem cells characteristics and epithelial-mesenchymal plasticity. HCMV presence parallels the succession of the observed cellular and molecular events potentially ensuing the transformation process. Correlation between PGCCs detection and HCMV presence in breast cancer tissue further validates our hypothesis in vivo. Our study indicates that some clinical HCMV strains conserve the potential to transform HMECs and fit with a “blastomere-like” model of oncogenesis, which may be relevant in the pathophysiology of breast cancer and other adenocarcinoma, especially of poor prognosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information files and from the corresponding authors on request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Dimri G, Band H, Band V. Mammary epithelial cell transformation: insights from cell culture and mouse models. Breast Cancer Res. 2005;7:171–9.
Polyak K. Heterogeneity in breast cancer. J Clin Investig. 2011;121:3786–8.
Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5:2929–43.
Hüsing A, Canzian F, Beckmann L, Garcia-Closas M, Diver WR, Thun MJ, et al. Prediction of breast cancer risk by genetic risk factors, overall and by hormone receptor status. J Med Genet. 2012;49:601–8.
Morales-Sánchez A, Fuentes-Pananá EM. Human viruses and cancer. Viruses. 2014;6:4047–79.
Coaquette A, Bourgeois A, Dirand C, Varin A, Chen W, Herbein G. Mixed cytomegalovirus glycoprotein B genotypes in immunocompromised patients. Clin Infect Dis. 2004;39:155–61.
Sinzger C, Digel M, Jahn G Cytomegalovirus Cell Tropism. In: Shenk TE, Stinski MF (eds). Human Cytomegalovirus. Springer-Verlag: Berlin-Heidelberg, 2008, pp 63–83.
Michaelis M, Doerr HW, Cinatl J. The story of human cytomegalovirus and cancer: increasing evidence and open questions. Neoplasia. 2009;11:1–9.
Cobbs CS, Harkins L, Samanta M, Gillespie GY, Bharara S, King PH, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62:3347–50.
Taher C, de Boniface J, Mohammad A-A, Religa P, Hartman J, Yaiw K-C, et al. High prevalence of human cytomegalovirus proteins and nucleic acids in primary breast cancer and metastatic sentinel lymph nodes. PLoS ONE. 2013;8:e56795.
Kumar A, Tripathy MK, Pasquereau S, Al Moussawi F, Abbas W, Coquard L, et al. The human cytomegalovirus strain DB activates oncogenic pathways in mammary epithelial cells. EBioMedicine. 2018;30:167–83.
Geder KM, Lausch R, O’Neill F, Rapp F. Oncogenic transformation of human embryo lung cells by human cytomegalovirus. Science. 1976;192:1134–7.
Niu N, Mercado-Uribe I, Liu J. Dedifferentiation into blastomere-like cancer stem cells via formation of polyploid giant cancer cells. Oncogene. 2017;36:4887–4900.
Zhang S, Mercado-Uribe I, Xing Z, Sun B, Kuang J, Liu J. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene. 2014;33:116–28.
Niu N, Zhang J, Zhang N, Mercado-Uribe I, Tao F, Han Z, et al. Linking genomic reorganization to tumor initiation via the giant cell cycle. Oncogenesis. 2016;5:e281.
Al Moussawi F, Kumar A, Pasquereau S, Tripathy MK, Karam W, Diab-Assaf M et al. The transcriptome of human mammary epithelial cells infected with the HCMV-DB strain displays oncogenic traits. Sci Rep. 2018;8. https://doi.org/10.1038/s41598-018-30109-1.
Collins-McMillen D, Rak M, Buehler JC, Igarashi-Hayes S, Kamil JP, Moorman NJ, et al. Alternative promoters drive human cytomegalovirus reactivation from latency. Proc Natl Acad Sci USA. 2019;116:17492–7.
Lee EYHP, Muller WJ. Oncogenes and Tumor Suppressor Genes. Cold Spring Harb Perspect Biol. 2010;2. https://doi.org/10.1101/cshperspect.a003236.
Prichard MN, Sztul E, Daily SL, Perry AL, Frederick SL, Gill RB, et al. Human cytomegalovirus UL97 kinase activity is required for the hyperphosphorylation of retinoblastoma protein and inhibits the formation of nuclear aggresomes. J Virol. 2008;82:5054–67.
Tsai HL, Kou GH, Chen SC, Wu CW, Lin YS. Human cytomegalovirus immediate-early protein IE2 tethers a transcriptional repression domain to p53. J Biol Chem. 1996;271:3534–40.
Knudsen ES, McClendon AK, Franco J, Ertel A, Fortina P, Witkiewicz AK. RB loss contributes to aggressive tumor phenotypes in MYC-driven triple negative breast cancer. Cell Cycle. 2015;14:109–22.
Zhao JJ, Gjoerup OV, Subramanian RR, Cheng Y, Chen W, Roberts TM, et al. Human mammary epithelial cell transformation through the activation of phosphatidylinositol 3-kinase. Cancer Cell. 2003;3:483–95.
Cojohari O, Peppenelli MA, Chan GC. Human cytomegalovirus induces an atypical activation of Akt to stimulate the survival of short-lived monocytes. J Virol. 2016;90:6443–52.
Luo J, Yan R, He X, He J. Constitutive activation of STAT3 and cyclin D1 overexpression contribute to proliferation, migration and invasion in gastric cancer cells. Am J Transl Res. 2017;9:5671–7.
Newbold RF. The significance of telomerase activation and cellular immortalization in human cancer. Mutagenesis. 2002;17:539–50.
Strååt K, Liu C, Rahbar A, Zhu Q, Liu L, Wolmer-Solberg N, et al. Activation of telomerase by human cytomegalovirus. J Natl Cancer Inst. 2009;101:488–97.
Romaniuk A, Paszel-Jaworska A, Totoń E, Lisiak N, Hołysz H, Królak A, et al. The non-canonical functions of telomerase: to turn off or not to turn off. Mol Biol Rep. 2019;46:1401–11.
White E. Mechanisms of apoptosis regulation by viral oncogenes in infection and tumorigenesis. Cell Death Differ. 2006;13:1371–7.
Xiong S, Feng Y, Cheng L. Cellular reprogramming as a therapeutic target in cancer. Trends Cell Biol. 2019;29:623–34.
Zheng L, Dai H, Zhou M, Li X, Liu C, Guo Z, et al. Polyploid cells rewire DNA damage response networks to overcome replication stress-induced barriers for tumour progression. Nat Commun. 2012;3:815.
McFarlane S, Nicholl MJ, Sutherland JS, Preston CM. Interaction of the human cytomegalovirus particle with the host cell induces hypoxia-inducible factor 1 alpha. Virology. 2011;414:83–90.
Weljie AM, Jirik FR. Hypoxia-induced metabolic shifts in cancer cells: Moving beyond the Warburg effect. Int J Biochem Cell Biol. 2011;43:981–9.
Yu Y, Clippinger AJ, Alwine JC. Viral affects on metabolism: changes in glucose and glutamine utilization during human cytomegalovirus infection. Trends Microbiol. 2011;19:360–7.
Yin X, Grove L, Datta NS, Long MW, Prochownik EV. C- myc overexpression and p53 loss cooperate to promote genomic instability. Oncogene. 1999;18:1177–84.
Hein SM, Haricharan S, Johnston AN, Toneff MJ, Reddy JP, Dong J, et al. Luminal epithelial cells within the mammary gland can produce basal cells upon oncogenic stress. Oncogene. 2016;35:1461–7.
Rodilla V, Fre S Cellular plasticity of mammary epithelial cells underlies heterogeneity of breast cancer. Biomedicines. 2018;6. https://doi.org/10.3390/biomedicines6040103.
Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature. 2013;503:218–23.
Yu W, Ma Y, Ochoa AC, Shankar S, Srivastava RK. Cellular transformation of human mammary epithelial cells by SATB2. Stem Cell Res. 2017;19:139–47.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
Liu J. The dualistic origin of human tumors. Semin Cancer Biol. 2018;53:1–16.
Liu J. The ‘life code’: a theory that unifies the human life cycle and the origin of human tumors. Semin Cancer Biol. 2020;60:380–97.
Odeberg J, Wolmer N, Falci S, Westgren M, Seiger Å, Söderberg-Nauclér C. Human cytomegalovirus inhibits neuronal differentiation and induces apoptosis in human neural precursor cells. J Virol. 2006;80:8929–39.
Luo MH, Hannemann H, Kulkarni AS, Schwartz PH, O’Dowd JM, Fortunato EA. Human cytomegalovirus infection causes premature and abnormal differentiation of human neural progenitor cells. J Virol. 2010;84:3528–41.
Fei F, Zhang D, Yang Z, Wang S, Wang X, Wu Z et al. The number of polyploid giant cancer cells and epithelial-mesenchymal transition-related proteins are associated with invasion and metastasis in human breast cancer. J Exp Clin Cancer Res. 2015;34. https://doi.org/10.1186/s13046-015-0277-8.
Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu Y, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2014;2:78–91.
Wang M-H, Sun R, Zhou X-M, Zhang M-Y, Lu J-B, Yang Y, et al. Epithelial cell adhesion molecule overexpression regulates epithelial-mesenchymal transition, stemness and metastasis of nasopharyngeal carcinoma cells via the PTEN/AKT/mTOR pathway. Cell Death Dis. 2018;9:2.
Yamashita N, Tokunaga E, Iimori M, Inoue Y, Tanaka K, Kitao H, et al. Epithelial paradox: clinical significance of coexpression of E-cadherin and vimentin with regard to invasion and metastasis of breast cancer. Clin Breast Cancer. 2018;18:e1003–e1009.
Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, Boumahdi S, et al. Identification of the tumour transition states occurring during EMT. Nature. 2018;556:463–8.
Høffding MK, Hyttel P. Ultrastructural visualization of the mesenchymal-to-epithelial transition during reprogramming of human fibroblasts to induced pluripotent stem cells. Stem Cell Res. 2015;14:39–53.
Oberstein A, Shenk T. Cellular responses to human cytomegalovirus infection: Induction of a mesenchymal-to-epithelial transition (MET) phenotype. PNAS. 2017;114:E8244–E8253.
Lopez-Sánchez LM, Jimenez C, Valverde A, Hernandez V, Peñarando J, Martinez A et al. CoCl2, a Mimic of Hypoxia, Induces Formation of Polyploid Giant Cells with Stem Characteristics in Colon Cancer. PLoS ONE. 2014;9. https://doi.org/10.1371/journal.pone.0099143.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–8.
Lawson DA, Bhakta NR, Kessenbrock K, Prummel KD, Yu Y, Takai K, et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature. 2015;526:131–5.
Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, et al. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507.
Tanabe K, Nakamura M, Narita M, Takahashi K, Yamanaka S. Maturation, not initiation, is the major roadblock during reprogramming toward pluripotency from human fibroblasts. Proc Natl Acad Sci USA. 2013;110:12172–9.
Ling G-Q, Chen D-B, Wang B-Q, Zhang L-S. Expression of the pluripotency markers Oct3/4, Nanog and Sox2 in human breast cancer cell lines. Oncol Lett. 2012;4:1264–8.
Huang Y-H, Luo M-H, Ni Y-B, Tsang JYS, Chan S-K, Lui PCW, et al. Increased SOX2 expression in less differentiated breast carcinomas and their lymph node metastases. Histopathology. 2014;64:494–503.
Wang D, Lu P, Zhang H, Luo M, Zhang X, Wei X, et al. Oct-4 and Nanog promote the epithelial-mesenchymal transition of breast cancer stem cells and are associated with poor prognosis in breast cancer patients. Oncotarget. 2014;5:10803–15.
Lu X, Mazur SJ, Lin T, Appella E, Xu Y. The pluripotency factor nanog promotes breast cancer tumorigenesis and metastasis. Oncogene. 2014;33:2655–64.
Aloia A, Petrova E, Tomiuk S, Bissels U, Déas O, Saini M. et al. The sialyl-glycolipid stage-specific embryonic antigen 4 marks a subpopulation of chemotherapy-resistant breast cancer cells with mesenchymal features. Breast Cancer Res. 2015;17. https://doi.org/10.1186/s13058-015-0652-6.
Chuang P-K, Hsiao M, Hsu T-L, Chang C-F, Wu C-Y, Chen B-R, et al. Signaling pathway of globo-series glycosphingolipids and β1,3-galactosyltransferase V (β3GalT5) in breast cancer. PNAS. 2019;116:3518–23.
Yu K-R, Yang S-R, Jung J-W, Kim H, Ko K, Han DW, et al. CD49f enhances multipotency and maintains stemness through the direct regulation of OCT4 and SOX2. Stem Cells. 2012;30:876–87.
Fornara O, Bartek J, Rahbar A, Odeberg J, Khan Z, Peredo I, et al. Cytomegalovirus infection induces a stem cell phenotype in human primary glioblastoma cells: prognostic significance and biological impact. Cell Death Differ. 2016;23:261–9.
Soroceanu L, Matlaf L, Khan S, Akhavan A, Singer E, Bezrookove V, et al. Cytomegalovirus Immediate-Early Proteins Promote Stemness Properties in Glioblastoma. Cancer Res. 2015;75:3065–76.
Jha HC, Banerjee S, Robertson ES The Role of Gammaherpesviruses in Cancer Pathogenesis. Pathogens. 2016;5. https://doi.org/10.3390/pathogens5010018.
Belzile J-P, Stark TJ, Yeo GW, Spector DH. Human cytomegalovirus infection of human embryonic stem cell-derived primitive neural stem cells is restricted at several steps but leads to the persistence of viral DNA. J Virol. 2014;88:4021–39.
Heieren MH, Kim YK, Balfour HH. Human cytomegalovirus infection of kidney glomerular visceral epithelial and tubular epithelial cells in culture. Transplantation. 1988;46:426–32.
Furukawa T. A variant of human cytomegalovirus derived from a persistently infected culture. Virology. 1984;137:191–4.
Coronel R, Takayama S, Juwono T, Hertel L. Dynamics of human cytomegalovirus infection in CD34+ hematopoietic cells and derived Langerhans-type dendritic cells. J Virol. 2015;89:5615–32.
Manners O, Murphy JC, Coleman A, Hughes DJ, Whitehouse A. Contribution of the KSHV and EBV lytic cycles to tumourigenesis. Curr Opin Virol. 2018;32:60–70.
Münz C. Latency and lytic replication in Epstein–Barr virus-associated oncogenesis. Nat Rev Microbiol. 2019;17:691–700.
Ganem D. KSHV and the pathogenesis of Kaposi sarcoma: listening to human biology and medicine. J Clin Investig. 2010;120:939–49.
Herbein G, Nehme Z. Polyploid giant cancer cells, a hallmark of oncoviruses and a new therapeutic challenge. Front Oncol. 2020;10:567116.
Khan KA, Coaquette A, Davrinche C, Herbein G. Bcl-3-regulated transcription from major immediate-early promoter of human cytomegalovirus in monocyte-derived macrophages. J Immunol. 2009;182:7784–94.
Lepiller Q, Abbas W, Kumar A, Tripathy MK, Herbein G. HCMV activates the IL-6-JAK-STAT3 axis in HepG2 cells and primary human hepatocytes. PLOS ONE. 2013;8:e59591.
Acknowledgements
This work was supported by grants from the University of Franche-Comté, and the Région Franche-Comté. Z.N. is a recipient of a doctoral scholarship from the municipality of Habbouch. The funders had no role in the data collection, analysis, patient recruitment, or decision to publish. We thank DImaCell Imaging Ressource Center, University of Bourgogne Franche-Comté, Faculty of Health Sciences, 25000 Besançon, France for technical support.
Author information
Authors and Affiliations
Contributions
ZN, SP, SHA, AC, CM, FM performed experiments. GH, M-PA., OA, MDA, JPF participated to the data analysis. GH conceived and designed the project. ZN, SP, JPF, GH wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Nehme, Z., Pasquereau, S., Haidar Ahmad, S. et al. Polyploid giant cancer cells, stemness and epithelial-mesenchymal plasticity elicited by human cytomegalovirus. Oncogene 40, 3030–3046 (2021). https://doi.org/10.1038/s41388-021-01715-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-021-01715-7
This article is cited by
-
Polyploidy and mTOR signaling: a possible molecular link
Cell Communication and Signaling (2024)
-
Generation of glioblastoma in mice engrafted with human cytomegalovirus-infected astrocytes
Cancer Gene Therapy (2024)
-
Polyploid giant cancer cells, cytokines and cytomegalovirus in breast cancer progression
Cancer Cell International (2023)
-
Molecular mechanism of vimentin nuclear localization associated with the migration and invasion of daughter cells derived from polyploid giant cancer cells
Journal of Translational Medicine (2023)
-
Polyploidy, EZH2 upregulation, and transformation in cytomegalovirus-infected human ovarian epithelial cells
Oncogene (2023)