Abstract
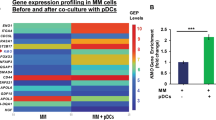
Bone marrow plasmacytoid dendritic cells (pDCs) in patients with multiple myeloma (MM) promote tumor growth, survival, drug resistance, and immune suppression. Understanding the molecular signaling crosstalk among the tumor cells, pDCs and immune cells will identify novel therapeutic approaches to enhance anti-MM immunity. Using oligonucleotide arrays, we found that pDC-MM interactions induce metabolic enzyme Alpha-Enolase (ENO1) in both pDCs and MM cells. Analysis of MM patient gene expression profiling database showed that ENO1 expression inversely correlates with overall survival. Protein expression analysis showed that ENO1 is expressed in pDC and MM cells; and importantly, that pDC-MM coculture further increases ENO1 expression in both MM cells and pDCs. Using our coculture models of patient autologous pDC-T-NK-MM cells, we examined whether targeting ENO1 can enhance anti-MM immunity. Biochemical inhibition of ENO1 with ENO1 inhibitor (ENO1i) activates pDCs, as well as increases pDC-induced MM-specific CD8+ CTL and NK cell activity against autologous tumor cells. Combination of ENO1i and anti-PD-L1 Ab or HDAC6i ACY-241 enhances autologous MM-specific CD8+ CTL activity. Our preclinical data therefore provide the basis for novel immune-based therapeutic approaches targeting ENO1, alone or in combination with anti-PD-L1 Ab or ACY241, to restore anti-MM immunity, enhance MM cytotoxicity, and improve patient outcome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Change history
07 September 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41388-021-01996-y
References
Anderson KC. The 39th David A. Karnofsky Lecture: bench-to-bedside translation of targeted therapies in multiple myeloma. J Clin Oncol. 2012;30:445–52.
Anderson KC. Therapeutic advances in relapsed or refractory multiple myeloma. J Natl Compr Canc Netw. 2013;11:676–9.
Anderson KC. Promise of immune therapies in multiple myeloma. J Oncol Pr. 2018;14:411–3.
Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–30.
Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–9.
Liberti MV, Locasale JW. Metabolism: a new layer of glycolysis. Nat Chem Biol. 2016;12:577–8. https://doi.org/10.1038/nchembio.2133.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 2009;324:1029–33. https://doi.org/10.1126/science.1160809.
Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014;513:559–63. https://doi.org/10.1038/nature13490.
Milosevic M, Fyles A, Hedley D, Hill R. The human tumor microenvironment: invasive (needle) measurement of oxygen and interstitial fluid pressure. Semin Radiat Oncol. 2004;14:249–58.
Kaelin WG Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008;30:393–402. https://doi.org/10.1016/j.molcel.2008.04.009.
Maiso P, Huynh D, Moschetta M, Sacco A, Aljawai Y, Mishima Y, et al. Metabolic signature identifies novel targets for drug resistance in multiple myeloma. Cancer Res 2015;75:2071–82. https://doi.org/10.1158/0008-5472.CAN-14-3400.
Ikeda S, Kitadate A, Abe F, Takahashi N, Tagawa H. Hypoxia-inducible KDM3A addiction in multiple myeloma. Blood Adv 2018;2:323–34. https://doi.org/10.1182/bloodadvances.2017008847.
Ader I, Brizuela L, Bouquerel P, Malavaud B, Cuvillier O. Sphingosine kinase 1: a new modulator of hypoxia inducible factor 1alpha during hypoxia in human cancer cells. Cancer Res 2008;68:8635–42. https://doi.org/10.1158/0008-5472.CAN-08-0917.
Shin YC, Joo CH, Gack MU, Lee HR, Jung JU. Kaposi’s sarcoma-associated herpesvirus viral IFN regulatory factor 3 stabilizes hypoxia-inducible factor-1 alpha to induce vascular endothelial growth factor expression. Cancer Res 2008;68:1751–9. https://doi.org/10.1158/0008-5472.CAN-07-2766.
Chauhan D, Singh AV, Brahmandam M, Carrasco R, Bandi M, Hideshima T, et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell 2009;16:309–23.
Ray A, Tian Z, Das DS, Coffman RL, Richardson P, Chauhan D, et al. A novel TLR-9 agonist C792 inhibits plasmacytoid dendritic cell-induced myeloma cell growth and enhance cytotoxicity of bortezomib. Leukemia. 2014;28:1716–24.
Ray A, Das DS, Song Y, Richardson P, Munshi NC, Chauhan D, et al. Targeting PD1-PDL1 immune checkpoint in plasmacytoid dendritic cell interactions with T cells, natural killer cells and multiple myeloma cells. Leukemia 2015;29:1441–4.
Ray A, Das DS, Song Y, Hideshima T, Tai YT, Chauhan D, et al. Combination of a novel HDAC6 inhibitor ACY-241 and anti-PD-L1 antibody enhances anti-tumor immunity and cytotoxicity in multiple myeloma. Leukemia 2018;32:843–6.
Ray A, Song Y, Du T, Tai YT, Chauhan D, Anderson KC. Targeting tryptophan catabolic kynurenine pathway enhances antitumor immunity and cytotoxicity in multiple myeloma. Leukemia. 2019. https://doi.org/10.1038/s41375-019-0558-x.
Bi E, Li R, Bover LC, Li H, Su P, Ma X, et al. E-cadherin expression on multiple myeloma cells activates tumor-promoting properties in plasmacytoid DCs. J Clin Invest 2018;128:4821–31. https://doi.org/10.1172/JCI121421.
Cappello P, Principe M, Bulfamante S, Novelli F. Alpha-Enolase (ENO1), a potential target in novel immunotherapies. Front Biosci (Landmark Ed). 2017;22:944–59.
Granchi C, Fancelli D, Minutolo F. An update on therapeutic opportunities offered by cancer glycolytic metabolism. Bioorg Med Chem Lett 2014;24:4915–5. https://doi.org/10.1016/j.bmcl.2014.09.041.
Ryans K, Omosun Y, McKeithen DN, Simoneaux T, Mills CC, Bowen N, et al. The immunoregulatory role of alpha enolase in dendritic cell function during Chlamydia infection. BMC Immunol 2017;18:27 https://doi.org/10.1186/s12865-017-0212-1.
Pacella I, Piconese S. Immunometabolic checkpoints of treg dynamics: adaptation to microenvironmental opportunities and challenges. Front Immunol 2019;10:1889 https://doi.org/10.3389/fimmu.2019.01889.
Zhu X, Miao X, Wu Y, Li C, Guo Y, Liu Y, et al. ENO1 promotes tumor proliferation and cell adhesion mediated drug resistance (CAM-DR) in Non-Hodgkin’s Lymphomas. Exp Cell Res 2015;335:216–23. https://doi.org/10.1016/j.yexcr.2015.05.020.
Barlogie B, Tricot G, Anaissie E, Shaughnessy J, Rasmussen E, van Rhee F, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N. Engl J Med. 2006;354:1021–30.
Muller FL, Colla S, Aquilanti E, Manzo VE, Genovese G, Lee J, et al. Passenger deletions generate therapeutic vulnerabilities in cancer. Nature 2012;488:337–42. https://doi.org/10.1038/nature11331
Stessman HA, Baughn LB, Sarver A, Xia T, Deshpande R, Mansoor A, et al. Profiling bortezomib resistance identifies secondary therapies in a mouse myeloma model. Mol Cancer Ther 2013;12:1140–50. https://doi.org/10.1158/1535-7163.MCT-12-1151.
Shaughnessy JD Jr, Zhan F, Burington BE, Huang Y, Colla S, Hanamura I, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood 2007;109:2276–84.
Vande Broek I, Leleu X, Schots R, Facon T, Vanderkerken K, Van Camp B, et al. Clinical significance of chemokine receptor (CCR1, CCR2 and CXCR4) expression in human myeloma cells: the association with disease activity and survival. Haematologica 2006;91:200–6.
Heuck CJ, Qu P, van Rhee F, Waheed S, Usmani SZ, Epstein J, et al. Five gene probes carry most of the discriminatory power of the 70-gene risk model in multiple myeloma. Leukemia 2014;28:2410–3. https://doi.org/10.1038/leu.2014.232.
Cappello P, Tonoli E, Roberta Curto R, Giordano D, Giovarelli M, Novelli F. Anti-a-enolase antibody limits the invasion of myeloid-derived suppressor cells and attenuates their restraining effector T cell response. Oncoimmunology. 2016;5:e1112940 https://doi.org/10.1080/2162402X.2015.1112940.
Manasanch EE, Han G, Mathur R, Qing Y, Zhang Z, Lee H, et al. A pilot study of pembrolizumab in smoldering myeloma: report of the clinical, immune, and genomic analysis. Blood Adv 2019;3:2400–8. https://doi.org/10.1182/bloodadvances.2019000300
Qorraj M, Bruns H, Böttcher M, Weigand L, Saul D, Mackensen A, et al. The PD-1/PD-L1 axis contributes to immune metabolic dysfunctions of monocytes in chronic lymphocytic leukemia. Leukemia 2017;31:470–8. https://doi.org/10.1038/leu.2016.214.
Li W, Sun Z. Mechanism of Action for HDAC Inhibitors-Insights from Omics Approaches. Int J Mol Sci. 2019;20:E1616 https://doi.org/10.3390/ijms20071616.
Lu C, Zhang K, Zhang Y, Tan M, Li Y, He X, et al. Preparation and characterization of vorinostat-coated beads for profiling of novel target proteins. J Chromatogr A 2014;1372C:34–41. https://doi.org/10.1016/j.chroma.2014.10.098.
Bae J, Hideshima T, Tai YT, Song Y, Richardson P, Raje N, et al. Histone deacetylase (HDAC) inhibitor ACY241 enhances anti-tumor activities of antigen-specific central memory cytotoxic T lymphocytes against multiple myeloma and solid tumors. Leukemia 2018;32:1932–47. https://doi.org/10.1038/s41375-018-0062-8.
Gu Z, Xia J, Xu H, Frech I, Tricot G, Zhan F. NEK2 promotes aerobic glycolysis in multiple myeloma through regulating splicing of pyruvate kinase. J Hematol Oncol. 2017;10:17 https://doi.org/10.1186/s13045-017-0392-4.
Zhao M, Fang W, Wang Y, Guo S, Shu L, Wang L, et al. Enolase-1 is a therapeutic target in endometrial carcinoma. Oncotarget 2015;6:15610–27.
Jung DW, Kim WH, Park SH, Lee J, Kim J, Su D, et al. A unique small molecule inhibitor of enolase clarifies its role in fundamental biological processes. ACS Chem Biol 2013;8:1271–82.
Chauhan D, Li G, Auclair D, Hideshima T, Richardson P, Podar K, et al. Identification of genes regulated by 2-methoxyestradiol (2ME2) in multiple myeloma cells using oligonucleotide arrays. Blood 2003;101:3606–14.
Acknowledgements
The grant supports for this investigation were provided by “Dr. Miriam and Sheldon Adelson Medical Research Foundation”, and by the National Institutes of Health Specialized Programs of Research Excellence (SPORE) grant P50100707, R01CA207237, and RO1 CA050947. KCA is an American Cancer Society Clinical Research Professor.
Author information
Authors and Affiliations
Contributions
D.C. conceptualized the project, designed research, analyzed data, and wrote the paper; A.R. designed research, performed the experiments, and analyzed the data; Y.S. and T.D. helped in flow cytometry; and K.C.A. provided clinical samples, and reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
K.C.A. is on Advisory board of Celgene, Millenium-Takeda, Gilead, Janssen, and Bristol Myers Squibb, and is a Scientific Founder with financial interest in Acetylon, Oncopep and C4 Therapeutics. D.C. is consultant to Stemline Therapeutic, Inc., Oncopeptides AB; and Equity owner in C4 Therapeutics. The remaining authors declare no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ray, A., Song, Y., Du, T. et al. Preclinical validation of Alpha-Enolase (ENO1) as a novel immunometabolic target in multiple myeloma. Oncogene 39, 2786–2796 (2020). https://doi.org/10.1038/s41388-020-1172-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-1172-0
This article is cited by
-
Identification and validation of serum autoantibodies in children with B-cell acute lymphoblastic leukemia by serological proteome analysis
Proteome Science (2022)
-
FOXM1 regulates glycolysis and energy production in multiple myeloma
Oncogene (2022)
-
Glioblastoma glycolytic signature predicts unfavorable prognosis, immunological heterogeneity, and ENO1 promotes microglia M2 polarization and cancer cell malignancy
Cancer Gene Therapy (2022)
-
Identification and validation of ecto-5' nucleotidase as an immunotherapeutic target in multiple myeloma
Blood Cancer Journal (2022)
-
Alpha-enolase (ENO1), identified as an antigen to monoclonal antibody 12C7, promotes the self-renewal and malignant phenotype of lung cancer stem cells by AMPK/mTOR pathway
Stem Cell Research & Therapy (2021)