Abstract
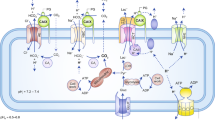
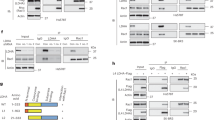
Tumor cells rely on glycolysis to meet their elevated demand for energy. Thereby they produce significant amounts of lactate and protons, which are exported via monocarboxylate transporters (MCTs), supporting the formation of an acidic microenvironment. The present study demonstrates that carbonic anhydrase IX (CAIX), one of the major acid/base regulators in cancer cells, forms a protein complex with MCT1 and MCT4 in tissue samples from human breast cancer patients, but not healthy breast tissue. Formation of this transport metabolon requires binding of CAIX to the Ig1 domain of the MCT1/4 chaperon CD147 and is required for CAIX-mediated facilitation of MCT1/4 activity. Application of an antibody, directed against the CD147-Ig1 domain, displaces CAIX from the transporter and suppresses CAIX-mediated facilitation of proton-coupled lactate transport. In cancer cells, this “metabolon disruption” results in a decrease in lactate transport, reduced glycolysis, and ultimately reduced cell proliferation. Taken together, the study shows that carbonic anhydrases form transport metabolons with acid/base transporters in human tumor tissue and that these interactions can be exploited to interfere with tumor metabolism and proliferation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Johnson JM, Cotzia P, Fratamico R, Mikkilineni L, Chen J, Colombo D, et al. MCT1 in invasive ductal carcinoma: monocarboxylate metabolism and aggressive breast cancer. Front Cell Dev Biol. 2017;5:27.
Bröer S, Rahman B, Pellegri G, Pellerin L, Martin JL, Verleysdonk S, et al. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J Biol Chem. 1997;272:30096–102.
Bröer S, Schneider H-P, Bröer A, Rahman B, Hamprecht B, Deitmer JW. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem J. 1998;333:167–74.
Dimmer KS, Friedrich B, Lang F, Deitmer JW, Bröer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350:219–27.
Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343:281–99.
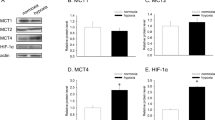
Pinheiro C, Albergaria A, Paredes J, Sousa B, Dufloth R, Vieira D, et al. Monocarboxylate transporter 1 is up-regulated in basal-like breast carcinoma. Histopathology. 2010;56:860–7.
Pinheiro C, Reis RM, Ricardo S, Longatto-Filho A, Schmitt F, Baltazar F. Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. J Biomed Biotechnol. 2010;2010:427694.
Choi J, Kim DH, Jung WH, Koo JS. Metabolic interaction between cancer cells and stromal cells according to breast cancer molecular subtype. Breast Cancer Res. 2013;15:78.
Kim S, Jung WH, Koo JS. The expression of Glut-1, CAIX, and MCT4 in mucinous carcinoma. J Breast Cancer. 2013;16:146.
Kwon JE, Jung W-H, Koo JS. The expression of metabolism-related proteins in phyllodes tumors. Tumor Biol. 2013;34:115–24.
Luz M, Perez M, Azzalis L, Sousa L, Adami F, Fonseca F, et al. Evaluation of MCT1, MCT4 and CD147 genes in peripheral blood cells of breast cancer patients and their potential use as diagnostic and prognostic markers. Int J Mol Sci. 2017;18:170.
Wilson MC, Meredith D, Halestrap AP. Fluorescence resonance energy transfer studies on the interaction between the lactate transporter MCT1 and CD147 provide information on the topology and stoichiometry of the complex in situ. J Biol Chem. 2002;277:3666–72.
Wilson MC, Meredith D, Manning Fox JE, Manoharan C, Davies AJ, Halestrap AP. Basigin (CD147) is the target for organomercurial inhibition of monocarboxylate transporter isoforms 1 and 4: The ancillary protein for the insensitive MCT2 is embigin (gp70). J Biol Chem.2005;280:27213–21.
Manoharan C, Wilson MC, Sessions RB, Halestrap AP. The role of charged residues in the transmembrane helices of monocarboxylate transporter 1 and its ancillary protein basigin in determining plasma membrane expression and catalytic activity. Mol Membr Biol. 2006;23:486–98.
Fossum S, Mallett S, Barclay AN. The MRC OX‐47 antigen is a member of the immunoglobulin superfamily with an unusual transmembrane sequence. Eur J Immunol. 1991;21:671–9.
Muramatsu T. Basigin (CD147), a multifunctional transmembrane glycoprotein with various binding partners. J Biochem. 2016;159:481–90.
Nabeshima K, Iwasaki H, Koga K, Hojo H, Suzumiya J, Kikuchi M. Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol Int. 2006;56:359–67.
Liu M, Tsang JYS, Lee M, Ni Y-B, Chan S-K, Cheung S-Y, et al. CD147 expression is associated with poor overall survival in chemotherapy treated triple-negative breast cancer. J Clin Pathol. 2018;71:1007–14.
Walter M, Simanovich E, Brod V, Lahat N, Bitterman H, Rahat MA. An epitope-specific novel anti-EMMPRIN polyclonal antibody inhibits tumor progression. Oncoimmunology. 2016;5:1–12.
Kuang YH, Liu YJ, Tang LL, Wang SM, Yan GJ, Liao LQ. Plasma soluble cluster of differentiation 147 levels are increased in breast cancer patients and associated with lymph node metastasis and chemoresistance. Hong Kong Med J. 2018;24:252–60.
Chiche J, Ilc K, Laferrière J, Trottier E, Dayan F, Mazure NM, et al. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69:358–68.
Swietach P, Hulikova A, Vaughan-Jones RD, Harris AL. New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene. 2010;29:6509–21.
Parks SK, Chiche J, Pouysségur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13:611–23.
Pastorek J, Pastorekova S. Hypoxia-induced carbonic anhydrase IX as a target for cancer therapy: from biology to clinical use. Semin Cancer Biol. 2015;31:52–64.
Jamali S, Klier M, Ames S, Barros LF, McKenna R, Deitmer JW, et al. Hypoxia-induced carbonic anhydrase IX facilitates lactate flux in human breast cancer cells by non-catalytic function. Sci Rep. 2015;5:13605.
Ames S, Pastorekova S, Becker HM. The proteoglycan-like domain of carbonic anhydrase IX mediates non-catalytic facilitation of lactate transport in cancer cells. Oncotarget. 2018;9:27940–57.
Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–83.
Pastorekova S, Zavada J. Carbonic anhydrase IX (CA IX) as a potential target for cancer therapy. Caner Ther. 2004;2:245–62.
Pastorek J, Pastoreková S, Callebaut I, Mornon JP, Zelník V, Opavský R, et al. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and a putative helix-loop-helix DNA binding segment. Oncogene. 1994;9:2877–88.
Pinheiro C, Sousa B, Albergaria A, Paredes J, Dufloth R, Vieira D, et al. GLUT1 and CAIX expression profiles in breast cancer correlate with adverse prognostic factors and MCT1 overexpression. Histol Histopathol. 2011;26:1279–86.
Vermeulen JF, van Brussel ASA, van der Groep P, Morsink FHM, Bult P, van der Wall E, et al. Immunophenotyping invasive breast cancer: paving the road for molecular imaging. BMC Cancer. 2012;12:240.
Choi J, Jung WH, Koo JS. Metabolism-related proteins are differentially expressed according to the molecular subtype of invasive breast cancer defined by surrogate immunohistochemistry. Pathobiology. 2012;80:41–52.
Adams A, van Brussel AS, Vermeulen JF, Mali WP, van der Wall E, van Diest PJ, et al. The potential of hypoxia markers as target for breast molecular imaging - a systematic review and meta-analysis of human marker expression. BMC Cancer. 2013;13:538.
Currie MJ, Beardsley BE, Harris GC, Gunningham SP, Dachs GU, Dijkstra B, et al. Immunohistochemical analysis of cancer stem cell markers in invasive breast carcinoma and associated ductal carcinoma in situ: relationships with markers of tumor hypoxia and microvascularity. Hum Pathol. 2013;44:402–11.
Bane AL, Whelan TJ, Pond GR, Parpia S, Gohla G, Fyles AW, et al. Tumor factors predictive of response to hypofractionated radiotherapy in a randomized trial following breast conserving therapy. Ann Oncol. 2014;25:992–8.
Ozretic P, Alvir I, Sarcevic B, Vujaskovic Z, Rendic-Miocevic Z, Roguljic A, et al. Apoptosis regulator Bcl-2 is an independent prognostic marker for worse overall survival in triple-negative breast cancer patients. Int J Biol Markers. 2018;33:109–15.
Tan EY, Yan M, Campo L, Han C, Takano E, Turley H, et al. The key hypoxia regulated gene CAIX is upregulated in basal-like breast tumours and is associated with resistance to chemotherapy. Br J Cancer. 2009;100:405–11.
Beketic-Oreskovic L, Ozretic P, Rabbani ZN, Jackson IL, Sarcevic B, Levanat S, et al. Prognostic significance of carbonic anhydrase IX (CA-IX), endoglin (CD105) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) in breast cancer patients. Pathol Oncol Res. 2011;17:593–603.
Srere PA. The metabolon. Trends Biochem Sci. 1985;10:109–10.
Srere PA. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124.
Deitmer JW, Becker HM. Transport metabolons with carbonic anhydrases. Front Physiol. 2013;4:291.
Pinheiro C, Longatto-Filho A, Azevedo-Silva J, Casal M, Schmitt FC, Baltazar F. Role of monocarboxylate transporters in human cancers: state of the art. J Bioenerg Biomembr. 2012;44:127–39.
Forero-Quintero LS, Ames S, Schneider H-P, Thyssen A, Boone CD, Andring JT, et al. Membrane-anchored carbonic anhydrase IV interacts with monocarboxylate transporters via their chaperones CD147 and GP70. J Biol Chem. 2018;294:593–607.
Alvarez BV, Vilas GL, Casey JR. Metabolon disruption: a mechanism that regulates bicarbonate transport. EMBO J. 2005;24:2499–511.
Ovens MJ, Davies AJ, Wilson MC, Murray CM, Halestrap AP. AR-C155858 is a potent inhibitor of monocarboxylate transporters MCT1 and MCT2 that binds to an intracellular site involving transmembrane helices 7–10. Biochem J. 2010;425:523–30.
Benjamin D, Robay D, Hindupur SK, Pohlmann J, Colombi M, El-Shemerly MY, et al. Dual inhibition of the lactate transporters MCT1 and MCT4 is synthetic lethal with metformin due to NAD+ depletion in cancer cells. Cell Rep. 2018;25:3047–58.
Chen C-L, Chu J-S, Su W-C, Huang S-C, Lee W-Y. Hypoxia and metabolic phenotypes during breast carcinogenesis: expression of HIF-1alpha, GLUT1, and CAIX. Virchows Arch. 2010;457:53–61.
Choi JH, Lim I, Noh WC, Kim H-A, Seong M-K, Jang S, et al. Prediction of tumor differentiation using sequential PET/CT and MRI in patients with breast cancer. Ann Nucl Med. 2018;32:389–97.
Cipolla V, Santucci D, Guerrieri D, Drudi FM, Meggiorini ML, De Felice C. Correlation between 3T apparent diffusion coefficient values and grading of invasive breast carcinoma. Eur J Radio. 2014;83:2144–50.
Belli P, Costantini M, Bufi E, Giardina GG, Rinaldi P, Franceschini G, et al. Diffusion magnetic resonance imaging in breast cancer characterisation: correlations between the apparent diffusion coefficient and major prognostic factors. Radio Med. 2015;120:268–76.
Swietach P. What is pH regulation, and why do cancer cells need it? Cancer Metastasis Rev. 2019;38:5–15.
Becker HM, Klier M, Schüler C, McKenna R, Deitmer JW. Intramolecular proton shuttle supports not only catalytic but also noncatalytic function of carbonic anhydrase II. Proc Natl Acad Sci USA. 2011;108:3071–6.
Noor SI, Pouyssegur J, Deitmer JW, Becker HM. Integration of a ‘proton antenna’ facilitates transport activity of the monocarboxylate transporter MCT4. FEBS J. 2017;284:149–62.
Noor SI, Jamali S, Ames S, Langer S, Deitmer JW, Becker HM. A surface proton antenna in carbonic anhydrase II supports lactate transport in cancer cells. Elife. 2018;7:1–31.
Hiremath SA, Surulescu C, Jamali S, Ames S, Deitmer JW, Becker HM. Modeling of pH regulation in tumor cells: direct interaction between proton-coupled lactate transporters and cancer-associated carbonicanhydrase. Math Biosci Eng. 2016;16:320–37.
Ädelroth P, Brzezinski P. Surface-mediated proton-transfer reactions in membrane-bound proteins. Biochim Biophys Acta—Bioenerg. 2004;1655:102–15.
Friedman R, Nachliel E, Gutman M. Molecular dynamics of a protein surface: ion-residues interactions. Biophys J. 2005;89:768–81.
Gutman M, Nachliel E, Friedman R. The dynamics of proton transfer between adjacent sites. Photochem Photobio Sci. 2006;5:531–7.
Stridh MH, Alt MD, Wittmann S, Heidtmann H, Aggarwal M, Riederer B, et al. Lactate flux in astrocytes is enhanced by a non-catalytic action of carbonic anhydrase II. J Physiol. 2012;590:2333–51.
Noor SI, Dietz S, Heidtmann H, Boone CD, McKenna R, Deitmer JW, et al. Analysis of the binding moiety mediating the interaction between monocarboxylate transporters and carbonic anhydrase II. J Biol Chem. 2015;290:4476–86.
Innocenti A, Pastorekova S, Pastorek J, Scozzafava A, De Simone G, Supuran CT. The proteoglycan region of the tumor-associated carbonic anhydrase isoform IX acts as anintrinsic buffer optimizing CO2 hydration at acidic pH values characteristic of solid tumors. Bioorg Med Chem Lett. 2009;19:5825–8.
Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites. 2017;7:48.
Mboge MY, Chen Z, Khokhar D, Wolff A, Ai L, Heldermon CD, et al. A non-catalytic function of carbonic anhydrase IX contributes to the glycolytic phenotype and pH regulation in human breast cancer cells. Biochem J. 2018;476:1497–513.
Renner K, Bruss C, Schnell A, Koehl G, Becker HM, Fante M, et al. Restricting glycolysis preserves T Cell effector functions and augments checkpoint therapy. Cell Rep. 2019;29:135–50.
Guan X, Bryniarski MA, Morris ME. In vitro and in vivo efficacy of the monocarboxylate transporter 1 inhibitor AR-C155858 in the murine 4T1 breast cancer tumor model. AAPS J. 2019;21:3.
Guan X, Rodriguez-Cruz V, Morris ME. Cellular uptake of MCT1 inhibitors AR-C155858 and AZD3965 and their effects on MCT-mediated transport of L-lactate in murine 4T1 breast tumor cancer cells. AAPS J. 2019;21:13.
Halestrap AP. The SLC16 gene family-structure, role and regulation in health and disease. Mol Asp Med. 2013;34:337–49.
Bonen A. Lactate transporters (MCT proteins) in heart and skeletal muscles. Med Sci Sports Exerc. 2000;32:778–89.
Debernardi R, Pierre K, Lengacher S, Magistretti PJ, Pellerin L. Cell-specific expression pattern of monocarboxylate transporters in astrocytes and neurons observed in different mouse brain cortical cell cultures. J Neurosci Res. 2003;73:141–55.
Jackson VN, Halestrap AP. The kinetics, substrate, and inhibitor specificity of the monocarboxylate (lactate) transporter of rat liver cells determined using the fluorescent intracellular pH indicator, 2’,7’-bis(carboxyethyl)-5(6)-carboxyfluorescein. J Biol Chem. 1996;271:861–8.
Zatovicova M, Tarábková K, Svastova E, Gibadulinová A, Mucha V, Jakubícková L, et al. Monoclonal antibodies generated in carbonic anhydrase IX-deficient mice recognize different domains of tumour-associated hypoxia-induced carbonic anhydrase IX. J Immunol Methods. 2003;282:117–34.
Klier M, Schüler C, Halestrap AP, Sly WS, Deitmer JW, Becker HM. Transport activity of the high-affinity monocarboxylate transporter MCT2 is enhanced by extracellular carbonic anhydrase IV but not by intracellular carbonic anhydrase II. J Biol Chem. 2011;286:27781–91.
Becker HM, Bröer S, Deitmer JW. Facilitated lactate transport by MCT1 when coexpressed with the sodium bicarbonate cotransporter (NBC) in Xenopus oocytes. Biophys J. 2004;86:235–47.
Becker HM. Transport of lactate: characterization of the transporters involved in transport at the plasma membrane by heterologous protein expression in Xenopus oocytes. Neuromethods. 2014;90:25–43.
Deitmer JW. Electrogenic sodium-dependent bicarbonate secretion by glial cells of the leech central nervous system. J Gen Physiol. 1991;98:637–55.
San Martín A, Ceballo S, Ruminot I, Lerchundi R, Frommer WB, Barros LF. A genetically encoded FRET lactate sensor and its use to detect the Warburg effect in single cancer cells. PLoS ONE. 2013;8:e57712.
Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, et al. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–21.
Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr Sect D Biol Crystallogr. 2004;60:2126–32.
Yu XL, Hu T, Du JM, Ding JP, Yang XM, Zhang J, et al. Crystal structure of HAb18G/CD147: implications for immunoglobulin superfamily homophilic adhesion. J Biol Chem. 2008;283:18056–65.
Pinard MA, Aggarwal M, Mahon BP, Tu C, McKenna R. A sucrose-binding site provides a lead towards an isoform-specific inhibitor of the cancer-associated enzyme carbonic anhydrase IX. Acta Crystallogr Sect Struct Biol Commun. 2015;71:1352–8.
Acknowledgements
We thank Heike Kanapin, Sandra Pfeiffer, Hans-Peter Schneider, and Patrick Pattar for technical assistance. The work was funded by the Deutsche Forschungsgemeinschaft (to H.M.B.; BE 4310/6–1), the Research Initiative BioComp (to H.M.B), and by stipends from the Lotto-Stiftung Rheinland-Pfalz and the FAZIT Stiftung (to S.A.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ames, S., Andring, J.T., McKenna, R. et al. CAIX forms a transport metabolon with monocarboxylate transporters in human breast cancer cells. Oncogene 39, 1710–1723 (2020). https://doi.org/10.1038/s41388-019-1098-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-019-1098-6
This article is cited by
-
Longitudinal FRET Imaging of Glucose and Lactate Dynamics and Response to Therapy in Breast Cancer Cells
Molecular Imaging and Biology (2022)
-
Understanding metabolic alterations and heterogeneity in cancer progression through validated immunodetection of key molecular components: a case of carbonic anhydrase IX
Cancer and Metastasis Reviews (2021)
-
Carbonic anhydrase IX and acid transport in cancer
British Journal of Cancer (2020)