Abstract
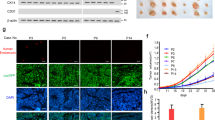
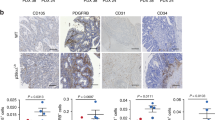
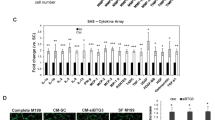
EGFL6, a member of the EGF-like superfamily, plays an important role during embryonic development and has been implicated in promotion of tumor angiogenesis without affecting wound healing. There is very little known about the function of EGFL6 in cancer cells. Here, we investigated whether EGFL6 plays a direct role in cancer cells in addition to the promotion of tumor angiogenesis. Our study showed that EGFL6 promoted epithelial–mesenchymal transition (EMT) and stemness of breast cancer cells and increased cell migration and invasion in cell culture studies. We also found that EGFL6 reduced apoptotic signaling in cancer cells and promoted tumor growth in vivo. Importantly, expression of EGFL6 in cancer cells and tumor endothelial cells not only increased tumor angiogenesis but also promoted migration of cancer cells. Such dual engagement of cancer and stromal cells suggests crosstalk mediated by EGFL6 in the tumor microenvironment. Blockade of EGFL6 using our novel anti-EGFL6 monoclonal antibody significantly reduced cancer cell migration, tumor angiogenesis, and tumor growth in mouse xenograft tumor models. Silencing EGFL6 mRNA by shRNA transfection of cancer cells also significantly reduced cancer cell migration, tumor angiogenesis, and tumor growth in mouse xenograft tumor models. Taken together, the results of this study indicate that targeting EGFL6 is a unique strategy for inhibiting both cancer cell metastasis and tumor angiogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Yeung G, Mulero JJ, Berntsen RP, Loeb DB, Drmanac R, Ford JE. Cloning of a novel epidermal growth factor repeat containing gene EGFL6: expressed in tumor and fetal tissues. Genomics. 1999;62:304–7.
Mas VR, Maluf DG, Archer KJ, Yanek KC, Fisher RA. Angiogenesis soluble factors as hepatocellular carcinoma noninvasive markers for monitoring hepatitis C virus cirrhotic patients awaiting liver transplantation. Transplantation. 2007;84:1262–71.
Noh K, Mangala LS, Han HD, Zhang N, Pradeep S, Wu SY, et al. Differential effects of EGFL6 on tumor versus wound angiogenesis. Cell Rep. 2017;21:2785–95.
Bai S, Ingram P, Chen YC, Deng N, Pearson A, Niknafs Y, et al. EGFL6 regulates the asymmetric division, maintenance, and metastasis of ALDH+ ovarian cancer cells. Cancer Res. 2016;76:6396–409.
Larimer BM, Deutscher SL. Identification of a peptide from in vivo bacteriophage display with homology to EGFL6: a candidate tumor vasculature ligand in breast cancer. J Mol Biomark Diagn. 2014;5:178.
Chim SM, Qin A, Tickner J, Pavlos N, Davey T, Wang H, et al. EGFL6 promotes endothelial cell migration and angiogenesis through the activation of extracellular signal-regulated kinase. J Biol Chem. 2011;286:22035–46.
Nichol D, Stuhlmann H. EGFL7: a unique angiogenic signaling factor in vascular development and disease. Blood. 2012;119:1345–52.
Nichol D, Shawber C, Fitch MJ, Bambino K, Sharma A, Kitajewski J, et al. Impaired angiogenesis and altered Notch signaling in mice overexpressing endothelial Egfl7. Blood. 2010;116:6133–43.
Garcia-Carbonero R, van Cutsem E, Rivera F, Jassem J, Gore I Jr, Tebbutt N, et al. Randomized phase II trial of parsatuzumab (anti-EGFL7) or placebo in combination with FOLFOX and bevacizumab for first-line metastatic colorectal cancer. oncologist. 2017;22:1281.
von Pawel J, Spigel DR, Ervin T, Losonczy G, Barlesi F, Juhasz E, et al. Randomized phase II trial of parsatuzumab (anti-EGFL7) or placebo in combination with carboplatin, paclitaxel, and bevacizumab for first-line nonsquamous non-small cell lung cancer. Oncologist. 2018;23:654–e58.
Lu C, Bonome T, Li Y, Kamat AA, Han LY, Schmandt R, et al. Gene alterations identified by expression profiling in tumor-associated endothelial cells from invasive ovarian carcinoma. Cancer Res. 2007;67:1757–68.
Buckanovich RJ, Sasaroli D, O’Brien-Jenkins A, Botbyl J, Hammond R, Katsaros D, et al. Tumor vascular proteins as biomarkers in ovarian cancer. J Clin Oncol. 2007;25:852–61.
Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725–31.
Brabletz T. EMT and MET in metastasis: where are the cancer stem cells?. Cancer Cell. 2012;22:699–701.
Biddle A, Mackenzie IC. Cancer stem cells and EMT in carcinoma. Cancer Metastasis Rev. 2012;31:285–93.
Wang X, Gong Y, Wang D, Xie Q, Zheng M, Zhou Y, et al. Analysis of gene expression profiling in meningioma: deregulated signaling pathways associated with meningioma and EGFL6 overexpression in benign meningioma tissue and serum. PLoS One. 2012;7:e52707.
Wang Y, Shi J, Chai K, Ying X, Zhou BP. The role of Snail in EMT and tumorigenesis. Curr Cancer Drug Targets. 2013;13:963–72.
Dong LJ, Hsieh JC, Chung AE. Two distinct cell attachment sites in entactin are revealed by amino acid substitutions and deletion of the RGD sequence in the cysteine-rich epidermal growth factor repeat 2. J Biol Chem. 1995;270:15838–43.
Fujiwara H, Ferreira M, Donati G, Marciano DK, Linton JM, Sato Y, et al. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell . 2011;144:577–89.
Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, et al. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428:754–8.
Gong C, Fang J, Li G, Liu HH, Liu ZS. Effects of microRNA-126 on cell proliferation, apoptosis and tumor angiogenesis via the down-regulating ERK signaling pathway by targeting EGFL7 in hepatocellular carcinoma. Oncotarget. 2017;8:52527–42.
Wang FY, Kang CS, Wang-Gou SY, Huang CH, Feng CY, Li XJ. EGFL7 is an intercellular EGFR signal messenger that plays an oncogenic role in glioma. Cancer Lett. 2017;384:9–18.
Dudvarski Stankovic N, Bicker F, Keller S, Jones DT, Harter PN, Kienzle A, et al. EGFL7 enhances surface expression of integrin alpha5beta1 to promote angiogenesis in malignant brain tumors. EMBO Mol Med. 2018;10:e8420.
Papaioannou D, Shen C, Nicolet D, McNeil B, Bill M, Karunasiri M, et al. Prognostic and biological significance of the proangiogenic factor EGFL7 in acute myeloid leukemia. Proc Natl Acad Sci USA. 2017;114:E4641–E7.
Dhupkar P, Zhao H, Mujoo K, An Z, Zhang N. Crk II silencing down-regulates IGF-IR and inhibits migration and invasion of prostate cancer cells. Biochem Biophys Rep. 2016;8:382–8.
Salameh A, Fan X, Choi BK, Zhang S, Zhang N, An Z. HER3 and LINC00052 interplay promotes tumor growth in breast cancer. Oncotarget. 2017;8:6526–39.
Fan X, Brezski RJ, Deng H, Dhupkar PM, Shi Y, Gonzalez A, et al. A novel therapeutic strategy to rescue the immune effector function of proteolytically inactivated cancer therapeutic antibodies. Mol Cancer Ther. 2015;14:681–91.
Shi Y, Fan X, Meng W, Deng H, Zhang N, An Z. Engagement of immune effector cells by trastuzumab induces HER2/ERBB2 downregulation in cancer cells through STAT1 activation. Breast Cancer Res. 2014;16:R33.
Zhang S, Mukherjee S, Fan X, Salameh A, Mujoo K, Huang Z, et al. Novel association of DJ-1 with HER3 potentiates HER3 activation and signaling in cancer. Oncotarget. 2016;7:65758–69.
Acknowledgements
We thank Dr. Wei Xiong and Ms. Hui Deng for their technical assistance in cell culture and antibody preparation. We also want to thank Dr. Yunfei Wen and Dr. Prahlad Ram for their suggestions and discussion on data analysis during the manuscript preparation. We thank Dr. Georgina Salazar for her critical editing of the manuscript. This study was supported in part by Welch Foundation grant (AU-0042-20030616) to ZA, and Cancer Prevention and Research Institute of Texas (CPRIT) Grants (RP150230 and RP150551) to ZA and NZ. This research was also supported by NIH support (P50 CA217685) to AKS, the Frank McGraw Memorial Chair in Cancer Research, and the ACS Research Professor Award.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
These authors contributed equally: Jingnan An, Yi Du
Electronic supplementary material
Rights and permissions
About this article
Cite this article
An, J., Du, Y., Fan, X. et al. EGFL6 promotes breast cancer by simultaneously enhancing cancer cell metastasis and stimulating tumor angiogenesis. Oncogene 38, 2123–2134 (2019). https://doi.org/10.1038/s41388-018-0565-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0565-9
This article is cited by
-
miR-4739 promotes epithelial-mesenchymal transition and angiogenesis in “driver gene-negative” non-small cell lung cancer via activating the Wnt/β-catenin signaling
Cellular Oncology (2023)
-
Parallel single-cell and bulk transcriptome analyses reveal key features of the gastric tumor microenvironment
Genome Biology (2022)
-
A single-cell atlas of non-haematopoietic cells in human lymph nodes and lymphoma reveals a landscape of stromal remodelling
Nature Cell Biology (2022)
-
EGFL6 promotes colorectal cancer cell growth and mobility and the anti‐cancer property of anti-EGFL6 antibody
Cell & Bioscience (2021)
-
EGFL6 regulates angiogenesis and osteogenesis in distraction osteogenesis via Wnt/β-catenin signaling
Stem Cell Research & Therapy (2021)