Abstract
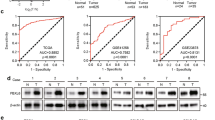
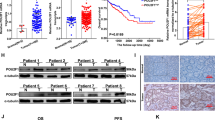
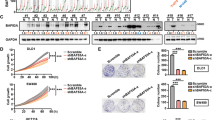
Aberrant expression of Forkhead box (FOX) transcription factors plays vital roles in carcinogenesis. However, the function of the FOX family member FOXC1 in maintenance of colorectal cancer (CRC) malignancy is unknown. Herein, FOXC1 expression in CRC specimens in The Cancer Genome Atlas (TCGA) cohort was analyzed and validated using immunohistochemistry with a tissue microarray. The effect of FOXC1 expression on proliferation of and glycolysis in CRC cells was assessed by altering its expression in vitro and in vivo. Mechanistic investigation was carried out using cell and molecular biological approaches. Our results showed that FOXC1 expression was higher in CRC specimens than in adjacent benign tissue specimens. Univariate survival analyses of the patients from whom the study specimens were obtained, and validated cohorts indicated that ectopic FOXC1 expression was significantly correlated with shortened survival. Silencing FOXC1 expression in CRC cells inhibited their proliferation and colony formation and decreased their glucose consumption and lactate production. In contrast, FOXC1 overexpression had the opposite effect. Furthermore, increased expression of FOXC1 downregulated that of a key glycolytic enzyme, fructose-1,6-bisphosphatase 1 (FBP1). Mechanistically, FOXC1 bound directly to the promoter regions of the FBP1 gene and negatively regulated its transcriptional activity. Collectively, aberrant FBP1 expression contributed to CRC tumorigenicity, and decreased FBP1 expression coupled with increased FOXC1 expression provided better prognostic information than did FOXC1 expression alone. Therefore, the FOXC1/FBP1 axis induces CRC cell proliferation, reprograms metabolism in CRCs, and constitutes potential prognostic predictors and therapeutic targets for CRC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2015;65:5–29.
Wu WK, Law PT, Lee CW, Cho CH, Fan D, Wu K, et al. MicroRNA in colorectal cancer: from benchtop to bedside. Carcinogenesis. 2011;32:247–53.
Cekaite L, Eide PW, Lind GE, Skotheim RI, Lothe RA. MicroRNAs as growth regulators, their function and biomarker status in colorectal cancer. Oncotarget. 2016;7:6476–505.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Martinez-Outschoorn UE, Peiris-Pages M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nat Rev Clin Oncol. 2017;14:11–31.
Weber GF. Time and circumstances: cancer cell metabolism at various stages of disease progression. Front Oncol. 2016;6:257.
Warburg O. On the origin of cancer cells. Science. 1956;123:309–14.
Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33.
Wechalekar K, Sharma B, Cook G. PET/CT in oncology—a major advance. Clin Radiol. 2005;60:1143–55.
Velpula KK, Bhasin A, Asuthkar S, Tsung AJ. Combined targeting of PDK1 and EGFR triggers regression of glioblastoma by reversing the Warburg effect. Cancer Res. 2013;73:7277–89.
Zhang Y, Yang JM. Altered energy metabolism in cancer: a unique opportunity for therapeutic intervention. Cancer Biol Ther. 2013;14:81–9.
Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo F, et al. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol. 2012;14:1295–304.
Marin-Hernandez A, Rodriguez-Enriquez S, Vital-Gonzalez PA, Flores-Rodriguez FL, Macias-Silva M, Sosa-Garrocho M, et al. Determining and understanding the control of glycolysis in fast-growth tumor cells. Flux control by an over-expressed but strongly product-inhibited hexokinase. FEBS J. 2006;273:1975–88.
Li B, Qiu B, Lee DS, Walton ZE, Ochocki JD, Mathew LK, et al. Fructose-1,6-bisphosphatase opposes renal carcinoma progression. Nature. 2014;513:251–5.
Dong C, Yuan T, Wu Y, Wang Y, Fan TW, Miriyala S, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23:316–31.
Liu X, Wang X, Zhang J, Lam EK, Shin VY, Cheng AS, et al. Warburg effect revisited: an epigenetic link between glycolysis and gastric carcinogenesis. Oncogene. 2010;29:442–50.
Zhang J, Wang J, Xing H, Li Q, Zhao Q, Li J. Down-regulation of FBP1 by ZEB1-mediated repression confers to growth and invasion in lung cancer cells. Mol Cell Biochem. 2016;411:331–40.
Zhu Y, Shi M, Chen H, Gu J, Zhang J, Shen B, et al. NPM1 activates metabolic changes by inhibiting FBP1 while promoting the tumorigenicity of pancreatic cancer cells. Oncotarget. 2015;6:21443–51.
Hirata H, Sugimachi K, Komatsu H, Ueda M, Masuda T, Uchi R, et al. Decreased expression of fructose-1,6-bisphosphatase associates with glucose metabolism and tumor progression in hepatocellular carcinoma. Cancer Res. 2016;76:3265–76.
Golson ML, Kaestner KH. Fox transcription factors: from development to disease. Development. 2016;143:4558–70.
Hannenhalli S, Kaestner KH. The evolution of Fox genes and their role in development and disease. Nat Rev Genet. 2009;10:233–40.
Li Q, Wu J, Wei P, Xu Y, Zhuo C, Wang Y, et al. Overexpression of forkhead Box C2 promotes tumor metastasis and indicates poor prognosis in colon cancer via regulating epithelial–mesenchymal transition. Am J Cancer Res. 2015;5:2022–34.
Weng W, Okugawa Y, Toden S, Toiyama Y, Kusunoki M, Goel A. FOXM1 and FOXQ1 are promising prognostic biomarkers and novel targets of tumor suppressive miR-342 in human colorectal cancer. Clin Cancer Res. 2016;22:4947–57.
Sun J, Li H, Huo Q, Cui M, Ge C, Zhao F, et al. The transcription factor FOXN3 inhibits cell proliferation by downregulating E2F5 expression in hepatocellular carcinoma cells. Oncotarget. 2016;7:43534–45.
Cui J, Shi M, Xie D, Wei D, Jia Z, Zheng S, et al. FOXM1 promotes the warburg effect and pancreatic cancer progression via transactivation of LDHA expression. Clin Cancer Res. 2014;20:2595–606.
Xia L, Huang W, Tian D, Zhu H, Qi X, Chen Z, et al. Overexpression of forkhead box C1 promotes tumor metastasis and indicates poor prognosis in hepatocellular carcinoma. Hepatology. 2013;57:610–24.
Paylakhi SH, Moazzeni H, Yazdani S, Rassouli P, Arefian E, Jaberi E, et al. FOXC1 in human trabecular meshwork cells is involved in regulatory pathway that includes miR-204, MEIS2, and ITGbeta1. Exp Eye Res. 2013;111:112–21.
Khan AO, Aldahmesh MA, Mohamed JY, Alkuraya FS. Congenital glaucoma with acquired peripheral circumferential iris degeneration. J AAPOS. 2013;17:105–7.
Ray PS, Wang J, Qu Y, Sim MS, Shamonki J, Bagaria SP, et al. FOXC1 is a potential prognostic biomarker with functional significance in basal-like breast cancer. Cancer Res. 2010;70:3870–6.
Xu ZY, Ding SM, Zhou L, Xie HY, Chen KJ, Zhang W, et al. FOXC1 contributes to microvascular invasion in primary hepatocellular carcinoma via regulating epithelial–mesenchymal transition. Int J Biol Sci. 2012;8:1130–41.
Wang L, Gu F, Liu CY, Wang RJ, Li J, Xu JY. High level of FOXC1 expression is associated with poor prognosis in pancreatic ductal adenocarcinoma. Tumour Biol. 2013;34:853–8.
Nagel S, Meyer C, Kaufmann M, Drexler HG, MacLeod RA. Deregulated FOX genes in Hodgkin lymphoma. Genes Chromosomes Cancer. 2014;53:917–33.
Wei LX, Zhou RS, Xu HF, Wang JY, Yuan MH. High expression of FOXC1 is associated with poor clinical outcome in non-small cell lung cancer patients. Tumour Biol. 2013;34:941–6.
Somerville TD, Wiseman DH, Spencer GJ, Huang X, Lynch JT, Leong HS, et al. Frequent derepression of the mesenchymal transcription factor gene FOXC1 in acute myeloid leukemia. Cancer Cell. 2015;28:329–42.
Huang W, Chen Z, Zhang L, Tian D, Wang D, Fan D, et al. Interleukin-8 induces expression of FOXC1 to promote transactivation of CXCR1 and CCL2 in hepatocellular carcinoma cell lines and formation of metastases in mice. Gastroenterology. 2015;149:1053–67 e14.
Aravalli RN, Greten TF. FoxC1: Novel regulator of inflammation-induced metastasis in hepatocellular carcinoma. Gastroenterology. 2015;149:861–3.
Jensen TW, Ray T, Wang J, Li X, Naritoku WY, Han B, et al. Diagnosis of basal-like breast cancer using a FOXC1-based assay. J Natl Cancer Inst. 2015;107.
Walenta S, Wetterling M, Lehrke M, Schwickert G, Sundfor K, Rofstad EK, et al. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000;60:916–21.
Pierrou S, Hellqvist M, Samuelsson L, Enerback S, Carlsson P. Cloning and characterization of seven human forkhead proteins: binding site specificity and DNA bending. EMBO J. 1994;13:5002–12.
Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–59.
Zhang N, Wei P, Gong A, Chiu WT, Lee HT, Colman H, et al. FoxM1 promotes beta-catenin nuclear localization and controls Wnt target-gene expression and glioma tumorigenesis. Cancer Cell. 2011;20:427–42.
Li D, Wei P, Peng Z, Huang C, Tang H, Jia Z, et al. The critical role of dysregulated FOXM1-PLAUR signaling in human colon cancer progression and metastasis. Clin Cancer Res. 2013;19:62–72.
Vakiani E, Janakiraman M, Shen R, Sinha R, Zeng Z, Shia J, et al. Comparative genomic analysis of primary versus metastatic colorectal carcinomas. J Clin Oncol. 2012;30:2956–62.
Kaneda H, Arao T, Tanaka K, Tamura D, Aomatsu K, Kudo K, et al. FOXQ1 is overexpressed in colorectal cancer and enhances tumorigenicity and tumor growth. Cancer Res. 2010;70:2053–63.
Wang J, Ray PS, Sim MS, Zhou XZ, Lu KP, Lee AV, et al. FOXC1 regulates the functions of human basal-like breast cancer cells by activating NF-kappaB signaling. Oncogene. 2012;31:4798–802.
Denko NC. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat Rev Cancer. 2008;8:705–13.
Kondaveeti Y, Guttilla Reed IK, White BA. Epithelial–mesenchymal transition induces similar metabolic alterations in two independent breast cancer cell lines. Cancer Lett. 2015;364:44–58.
Courtnay R, Ngo DC, Malik N, Ververis K, Tortorella SM, Karagiannis TC. Cancer metabolism and the Warburg effect: the role of HIF-1 and PI3K. Mol Biol Rep. 2015;42:841–51.
Dang CV The interplay between MYC and HIF in the Warburg effect. Ernst Schering Foundation Symp. Proc; 2007. p. 35–53.
Li D, Peng Z, Tang H, Wei P, Kong X, Yan D, et al. KLF4-mediated negative regulation of IFITM3 expression plays a critical role in colon cancer pathogenesis. Clin Cancer Res. 2011;17:3558–68.
Li Q, Wei P, Huang B, Xu Y, Li X, Li Y, et al. MAEL expression links epithelial–mesenchymal transition and stem cell properties in colorectal cancer. Int J Cancer. 2016;139:2502–11.
Zhou A, Lin K, Zhang S, Chen Y, Zhang N, Xue J, et al. Nuclear GSK3beta promotes tumorigenesis by phosphorylating KDM1A and inducing its deubiquitylation by USP22. Nat Cell Biol. 2016;18:954–66.
Quan M, Cui J, Xia T, Jia Z, Xie D, Wei D, et al. Merlin/NF2 suppresses pancreatic tumor growth and metastasis by attenuating the FOXM1-mediated Wnt/beta-catenin signaling. Cancer Res. 2015;75:4778–89.
Guo K, Cui J, Quan M, Xie D, Jia Z, Wei D, et al. The Novel KLF4/MSI2 signaling pathway regulates growth and metastasis of pancreatic cancer. Clin Cancer Res. 2017;23:687–96.
Li Q, Qin Y, Wei P, Lian P, Li Y, Xu Y, et al. Gas1 inhibits metastatic and metabolic phenotypes in colorectal carcinoma. Mol Cancer Res. 2016;14:830–40.
Shi M, Cui J, Du J, Wei D, Jia Z, Zhang J, et al. A novel KLF4/LDHA signaling pathway regulates aerobic glycolysis in and progression of pancreatic cancer. Clin Cancer Res. 2014;20:4370–80.
Li Q, Qin Y, Wei P, Lian P, Li Y, Xu Y, et al. Gas1 inhibits metastatic and metabolic phenotypes in colorectal carcinoma. Mol Cancer Res. 2016;14:830–40.
Acknowledgements
We thank Don Norwood in the Department of Scientific Publications at The University of Texas MD Anderson Cancer Center for editorial assistance and Jianping Zhang at the Center for Biomedical Imaging, Fudan University, for assistance in the mouse PET/CT study.
Author contributions
Conception and design: D. Li, K. Xie. Development of methodology: Q. Li, Y. Li, D. Xie. Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): Q. Li,M. Zhang, S. Cai, D. Li, G. Li, P. Wei, Y. Li, Y. Xu, X. Li, D. Xie. Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): J Wu, P. Wei, Y. Li, D. Xie. Writing, review, and/or revision of the manuscript: Q. Li, J Wu, D. Li, K. Xie. Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): Q. Li, Y. Xu, X. Li, K. Xie. Study supervision: D. Li, P. Wei. Other (financial support): S. Cai, D. Li, K. Xie
Funding
This research was supported by grants from the National Science Foundation of China (81472222, 81702353, 81772583, 81572254, 81372646, 81302097, and 81672374) and Shanghai Rising-Star Program(17QA1400900), Shanghai Municipal Natural Science Foundation (17ZR1406400), Municipal Human Resources Development Program for Outstanding Young Talents in Medical and Health Sciences in Shanghai (2017YQ046), National Key Basic Research Program of China (2014CBA02002) and grants R01-CA129956, R01-CA148954, R01CA152309, R01CA172233, and R01CA195651 from the National Cancer Institute, National Institutes of Health.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Li, Q., Wei, P., Wu, J. et al. The FOXC1/FBP1 signaling axis promotes colorectal cancer proliferation by enhancing the Warburg effect. Oncogene 38, 483–496 (2019). https://doi.org/10.1038/s41388-018-0469-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0469-8
This article is cited by
-
Elevated galectin-3 levels detected in women with hyperglycemia during early and mid-pregnancy antagonizes high glucose − induced trophoblast cells apoptosis via galectin-3/foxc1 pathway
Molecular Medicine (2023)
-
Methyltransferase like 3 inhibition limits intrahepatic cholangiocarcinoma metabolic reprogramming and potentiates the efficacy of chemotherapy
Oncogene (2023)
-
The role of LINC01419 in regulating the cell stemness in lung adenocarcinoma through recruiting EZH2 and regulating FBP1 expression
Biology Direct (2022)
-
Overexpression of FOXC1 Promotes Tumor Metastasis by Activating the Wnt/β-Catenin Signaling Pathway in Gastric Cancer
Digestive Diseases and Sciences (2022)
-
Fructose-1,6-bisphosphatase 1 (FBP1) is an independent biomarker associated with a favorable prognosis in esophageal adenocarcinoma
Journal of Cancer Research and Clinical Oncology (2022)