Abstract
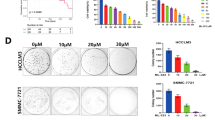
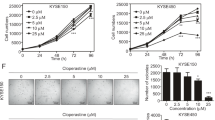
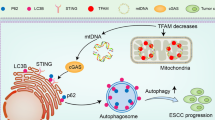
Esophageal squamous cell carcinoma (ESCC) is one of the malignancies in digestive system, with a low 5-year survival rate. We previously revealed that Sequestosome 1 (SQSTM1/p62) protein levels were upregulated in ESCC tissues. However, it is unclear about the function of p62 and the underlying mechanism. Here, we used immunofluorescence and immunohistochemistry to investigate the expression of p62 in ESCC. Western blotting, quantitative RT-PCR, colony formation assay, flow cytometry, immunoprecipitation and xenograft tumor assay were used to analyze the role of p62 in vitro and vivo. Here, we showed that p62 serves as a regulator of cell apoptosis under serum starvation condition in ESCC cells. Through activating the protein kinase C iota (PKCiota)—S-phase kinase-associated protein 2 (SKP2) signaling pathway, p62 enhances cell apoptosis resistance and colony formation in vitro and tumor growth in mouse models. Through interaction with the domains PB1, p62 upregulated the expression of PKCiota and then depressed the ubiquitin-mediated proteasomal degradation of SKP2. p62-silencing combined with a PKCiota inhibitor ATM significantly enhanced cell apoptosis and inhibited cell survival. Immunohistochemical analysis revealed a positive association between the expression of p62 and SKP2 in primary ESCC tissues. And importantly, p62 presented a markedly cytoplasmic translocation in cancerous cells, including in 16 (30.76%) tumors at stage T1, as compared with its nuclear location in normal esophageal epithelial cells. In summary, p62 plays an anti-apoptotic role in ESCC cells via stabilizing SKP2 under serum starvation condition. These data suggest that p62 might be an early biomarker and a candidate therapeutic target of ESCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–12.
Lu SH. Alterations of oncogenes and tumor suppressor genes in esophageal cancer in China. Mutat Res. 2000;462:343–53.
Wang BS, Yang Y, Lu HZ, Shang L, Zhang Y, Hao JJ, et al. Inhibition of atypical protein kinase Ciota induces apoptosis through autophagic degradation of beta-catenin in esophageal cancer cells. Mol Carcinog. 2014;53:514–25.
Johansen T, Lamark T. Selective autophagy mediated by autophagic adapter proteins. Autophagy. 2011;7:279–96.
Moscat J, Diaz-Meco MT. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell. 2009;137:1001–4.
Duran A, Hernandez ED, Reina-Campos M, Castilla EA, Subramaniam S, Raghunandan S, et al. p62/SQSTM1 by binding to vitamin D receptor inhibits hepatic stellate cell activity, fibrosis, and liver cancer. Cancer Cell. 2016;30:595–609.
Mohamed A, Ayman A, Deniece J, Wang T, Kovach C, Siddiqui MT, et al. P62/ubiquitin IHC expression correlated with clinicopathologic parameters and outcome in gastrointestinal carcinomas. Front Oncol. 2015;5:70.
Inoue D, Suzuki T, Mitsuishi Y, Miki Y, Suzuki S, Sugawara S, et al. Accumulation of p62/SQSTM1 is associated with poor prognosis in patients with lung adenocarcinoma. Cancer Sci. 2012;103:760–6.
Burdelski C, Reiswich V, Hube-Magg C, Kluth M, Minner S, Koop C, et al. Cytoplasmic accumulation of sequestosome 1 (p62) is a predictor of biochemical recurrence, rapid tumor cell proliferation, and genomic instability in prostate cancer. Clin Cancer Res. 2015;21:3471–9.
Li L, Shen C, Nakamura E, Ando K, Signoretti S, Beroukhim R, et al. SQSTM1 is a pathogenic target of 5q copy number gains in kidney cancer. Cancer Cell. 2013;24:738–50.
Luo RZ, Yuan ZY, Li M, Xi SY, Fu J, He J. Accumulation of p62 is associated with poor prognosis in patients with triple-negative breast cancer. Onco Targets Ther. 2013;6:883–8.
Inui T, Chano T, Takikita-Suzuki M, Nishikawa M, Yamamoto G, Okabe H. Association of p62/SQSTM1 excess and oral carcinogenesis. PLoS ONE. 2013;8:e74398.
Nakano T, Nakaso K, Nakashima K, Ohama E. Expression of ubiquitin-binding protein p62 in ubiquitin-immunoreactive intraneuronal inclusions in amyotrophic lateral sclerosis with dementia: analysis of five autopsy cases with broad clinicopathological spectrum. Acta Neuropathol. 2004;107:359–64.
Zhang MZ, Wang Y, Paueksakon P, Harris RC. Epidermal growth factor receptor inhibition slows progression of diabetic nephropathy in association with a decrease in endoplasmic reticulum stress and an increase in autophagy. Diabetes. 2014;63:2063–72.
He PX, Che YS, He QJ, Chen Y, Ding J. G226, a novel epipolythiodioxopiperazine derivative, induces autophagy and caspase-dependent apoptosis in human breast cancer cells in vitro. Acta Pharmacol Sin. 2014;35:1055–64.
Xie WY, Zhou XD, Li Q, Chen LX, Ran DH. Acid-induced autophagy protects human lung cancer cells from apoptosis by activating ER stress. Exp Cell Res. 2015;339:270–9.
Zhao Z, Wang H, Zhang L, Mei X, Hu J, Huang K. Receptor for advanced glycation end product blockade enhances the chemotherapeutic effect of cisplatin in tongue squamous cell carcinoma by reducing autophagy and modulating the Wnt pathway. Anticancer Drugs. 2017;28:187–96.
Zhang H, Kobayashi R, Galaktionov K, Beach D. p19Skp1 and p45Skp2 are essential elements of the cyclin A-CDK2 S phase kinase. Cell. 1995;82:915–25.
Wei W, Ayad NG, Wan Y, Zhang GJ, Kirschner MW, Kaelin WG,Jr. Degradation of the SCF component Skp2 in cell-cycle phase G1 by the anaphase-promoting complex. Nature. 2004;428:194–8.
Kopsiaftis S, Sullivan KL, Garg I, Taylor JA, Claffey KP. AMPKalpha2 regulates bladder cancer growth through SKP2-mediated degradation of p27. Mol Cancer Res. 2016;14:1182–94.
Yamada S, Yanamoto S, Naruse T, Matsushita Y, Takahashi H, Umeda M, et al. Skp2 regulates the expression of MMP-2 and MMP-9, and enhances the invasion potential of oral squamous cell carcinoma. Pathol Oncol Res. 2016;22:625–32.
Lu W, Liu S, Li B, Xie Y, Izban MG, Ballard BR, et al. SKP2 loss destabilizes EZH2 by promoting TRAF6-mediated ubiquitination to suppress prostate cancer. Oncogene. 2017;36:1364–73.
Xu D, Li CF, Zhang X, Zhang X, Gong Z, Chan CH, et al. Skp2-macroH2A1-CDK8 axis orchestrates G2/M transition and tumorigenesis. Nat Commun. 2015;6:6641.
Wang XC, Wu YP, Ye B, Lin DC, Feng YB, Zhang ZQ, et al. Suppression of anoikis by SKP2 amplification and overexpression promotes metastasis of esophageal squamous cell carcinoma. Mol Cancer Res. 2009;7:12–22.
Nakamura K, Johnson GL. PB1 domains of MEKK2 and MEKK3 interact with the MEK5 PB1 domain for activation of the ERK5 pathway. J Biol Chem. 2003;278:36989–92.
Nakamura K, Kimple AJ, Siderovski DP, Johnson GL. PB1 domain interaction of p62/sequestosome 1 and MEKK3 regulates NF-kappaB activation. J Biol Chem. 2010;285:2077–89.
Burke RM, Berk BC. The role of PB1 domain proteins in endothelial cell dysfunction and disease. Antioxid Redox Signal. 2015;22:1243–56.
Regala RP, Weems C, Jamieson L, Copland JA, Thompson EA, Fields AP. Atypical protein kinase Ciota plays a critical role in human lung cancer cell growth and tumorigenicity. J Biol Chem. 2005;280:31109–15.
Ren J, Wang J, Wang Z, Wu J. Structural and biochemical insights into the homotypic PB1-PB1 complex between PKCzeta and p62. Sci China Life Sci. 2014;57:69–80.
Marjuki H, Yen HL, Franks J, Webster RG, Pleschka S, Hoffmann E. Higher polymerase activity of a human influenza virus enhances activation of the hemagglutinin-induced Raf/MEK/ERK signal cascade. Virol J. 2007;4:134.
Fields AP, Frederick LA, Regala RP. Targeting the oncogenic protein kinase Ciota signalling pathway for the treatment of cancer. Biochem Soc Trans. 2007;35:996–1000.
Ma CQ, Yang Y, Wang JM, Du GS, Shen Q, Liu Y, et al. The aPKCiota blocking agent ATM negatively regulates EMT and invasion of hepatocellular carcinoma. Cell Death Dis. 2014;5:e1129.
Scharf VF, Farese JP, Siemann DW, Abbott JR, Kiupel M, Salute ME, et al. Effects of aurothiomalate treatment on canine osteosarcoma in a murine xenograft model. Anticancer Drugs. 2014;25:332–9.
Mansfield AS, Fields AP, Jatoi A, Qi Y, Adjei AA, Erlichman C, et al. Phase I dose escalation study of the PKCiota inhibitor aurothiomalate for advanced non-small-cell lung cancer, ovarian cancer, and pancreatic cancer. Anticancer Drugs. 2013;24:1079–83.
Pankiv S, Lamark T, Bruun JA, Overvatn A, Bjorkoy G, Johansen T. Nucleocytoplasmic shuttling of p62/SQSTM1 and its role in recruitment of nuclear polyubiquitinated proteins to promyelocytic leukemia bodies. J Biol Chem. 2010;285:5941–53.
Clausen TH, Lamark T, Isakson P, Finley K, Larsen KB, Brech A, et al. p62/SQSTM1 and ALFY interact to facilitate the formation of p62 bodies/ALIS and their degradation by autophagy. Autophagy. 2010;6:330–44.
Acknowledgements
This work was supported by National Natural Science Foundation of China (31571420 and 81520108023). Beijing Natural Science Foundation (7151008) and CAMS Innovation Fund for Medical Sciences (CIFMS2016-I2M-1-001/3-007).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Shi, C., Pan, BQ., Shi, F. et al. Sequestosome 1 protects esophageal squamous carcinoma cells from apoptosis via stabilizing SKP2 under serum starvation condition. Oncogene 37, 3260–3274 (2018). https://doi.org/10.1038/s41388-018-0217-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-018-0217-0