Abstract
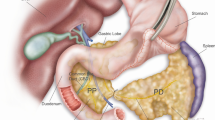
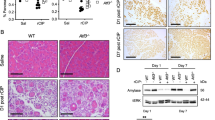
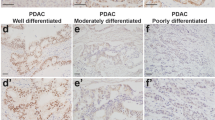
Pancreatic cancer (PDAC) is one of the most dismal of human malignancies. Inhibiting or delaying the progression of precursor lesions of PDAC, pancreatic intraepthial neoplasia (PanINs), to invasive cancer, would be a major step. In the present study, we used a transgenic murine model of pancreatic cancer to evaluate the impact of a conditional knockout of the transcription factor Snail1, a major factor in epithelial-to-mesenchymal transition, on acinar-to-ductal formation and on PanIN progression. By interbreeding conditional LsL-Snailfloxf/wt; LsL-KrasG12D and Pdx1-Cre strains, we obtained LsL-KrasG12D;Pdx1-Cre(KP) mice, Snail1 heterozygous knockout LsL-KrasG12D; LsL-Snailflox/-;Pdx1-Cre(KPShet) mice or Snail1 homozygous knockout LsL-KrasG12D;LsL-Snailflox/flox;Pdx1-Cre(KPS) mice. Mice were then followed in a longitudinal study for 2, 4, 6, 8, 10, and 12 months. Furthermore, in mice with a genetic or pharmacological inhibition of Snail1, using the Snail1 inhibitor GN25, a model of pancreatic injury by administration of cerulein was introduced to evaluate ADM formation in this setting. A translational approach with a tissue microarray (TMA) of human PanINs and an in vivo nude mouse platform to test GN25 in human pancreatic adenocarcinoma was then adopted. Quantification of PanINs showed delayed initiation and progression of PanIN lesions at all ages in both homozygous and heterozygous Snaildel1;Pdx-1-Cre;LSL-KrasG12D/+-Mice. PanINs at TMA revealed snail expression in the majority of cases. GN25 showed growth inhibition in 2/2 human pancreatic adenocarcinomas using a nude mice in vivo platform. Genetic and pharmacologic abrogation of Snail1 signaling in exocrine pancreas impairs development of acinar-to-ductal metaplasia following cerulein-mediated pancreatic injury. The present study suggests a fundamental new approach to delay the progression of PDAC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Haeno H, Gonen M, Davis MB, Herman JM, Iacobuzio-Donahue CA, Michor F. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell. 2011;148:362–75.
Rhim AD, Mirek ET, Aiello NM, Maitra A, Bailey JM, McAllister F, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–61.
Hezel AF, Kimmelman AC, Stanger BZ, Bardeesy N, Depinho RA. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2006;20:1218–49.
Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–7.
Feldmann G, Beaty R, Hruban RH, Maitra A. Molecular genetics of pancreatic intraepithelial neoplasia. J Hepatobiliary Pancreat Surg. 2007;14:224–32.
Kanda M, Matthaei H, Wu J, Hong SM, Yu J, Borges M, et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology. 2012;142:730–3.
Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–50.
Morris JP 4th, Cano DA, Sekine S, Wang SC, Hebrok M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest. 2010;120:508–20.
Kopp JL, von Figura G, Mayes E, Liu FF, Dubois CL, Morris JP 4th, et al. Identification of Sox9-dependent acinar-to-ductal reprogramming as the principal mechanism for initiation of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:737–50.
Fendrich V, Esni F, Garay MV, Feldmann G, Habbe N, Jensen JN, et al. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology. 2008;135:621–31.
Lowenfels AB, Maisonneuve P, Cavallini G, Ammann RW, Lankisch PG, Andersen JR, et al. Pancreatitis and the risk of pancreatic cancer. International Pancreatitis Study Group. N Engl J Med. 1993;328:1433–7.
Lim J, Thiery JP. Epithelial-mesenchymal transitions: Insights from development. Development. 2012;139:3471–86.
Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: Acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;b9:265–73.
Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83.
Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83.
Fendrich V, Chen NM, Neef M, Waldmann J, Bucholz M, Feldmann G, et al. The Angiotensin-I-converting enzyme inhibitor enalapril and aspirin delay progression of pancreatic intraepithelial neoplasia and cancer formation in a genetically engineered mouse model of pancreatic cancer. Gut. 2010;59:630–7.
Rubio-Viqueira B, Jimeno A, Cusatis G, Zhang X, Iacobuzio-Donahue C, Karikari C, et al. An in vivo platform for translational drug development in pancreatic cancer. Clin Cancer Res. 2006;12:4652–61.
Murray S, Carver EA, Gridley T. Generation of a Snail1 (Snai1) conditional null allele. Genesis. 2006;44:7–11.
Miyoshi A, Kitajima Y, Kido S, Shimonishi T, Matsuyama S, Kitahara K, et al. Snail accelerates cancer invasion by upregulating MMP expression and is associated with poor prognosis of hepatocellular carcinoma. Br J Cancer. 2005;92:252–8.
Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–30.
Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res. 2007;13:4769–76.
von Burstin J, Eser S, Paul MC, Seidler B, Brandl M, Messer M, et al. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology. 2009;137:361–71.
Barallo-Gimeno A, Nieto MA. The snail genes as inducers of cell movement and survival. Implications in development and cancer. Development. 2005;132:3151–61.
Yin T, Wang C, Liu T, Zhao G, Zha Y, Yang Y. Expression of snail in pancreatic cancer promotes metastasis and chemoresistance. J Surg Res. 2007;141:196–203.
Zhang S, Zhang XQ, Huang SL, Chen M, Shen SS, Ding XW, et al. The Effects of HSP27 on gemcitabine-resistant pancreatic cancer cell line through snail. Pancreas. 2015;44:1121–9.
Hruban RH, Adsay NV, Bores-Saavedra J. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: consensus report and recommendations. Cancer Res. 2006;66:95–106.
Taniguchi S, Iwamura T, Katsuki T. Correlation between spontaneous metastatic potential and type I collagenolytic activity in a human pancreatic cancer cell line (SUIT-2) and sublines. Clin Exp Metastas--. 1992;10:259–66.
Fendrich V, Wichmann S, Wiese D, Waldmann J, Lauth M, Rexin P, et al. Inhibition of heat shock protein 90 with AUY922 represses tumor growth in a transgenic mouse model of islet cell neoplasms. Neuroendocrinology. 2014;100:300–9.
Rhim AD. Epithelial to mesenchymal transition and the generation of stem-like cells in pancreatic cancer. Pancreatology. 2013;13:114–7.
Acknowledgements
Grant support: VF and ML were supported by a Research Grant from the Sander Stiftung
Author contributions
VF: Study concept and design, obtained funding, analysis and interpretation, drafting manuscript, FJ: acquisition of data, critical revision of the manuscript. SB: Acquisition of data, analysis. MA: Acquisition of data. ML: Obtained funding, technical support, acquisition of data, critical revision of the manuscript. FE: Technical support, acquisition of data. KH: Acquisition of data. JD: Acquisition of data. EPS: Critical revision of the manuscript for important intellectual content. JPNH: Critical revision of the manuscript for important intellectual content, AB: Acquisition of data. DKB: Critical revision of the manuscript for important intellectual content. JW: Study concept and design, analysis and interpretation, drafting the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Fendrich, V., Jendryschek, F., Beeck, S. et al. Genetic and pharmacologic abrogation of Snail1 inhibits acinar-to-ductal metaplasia in precursor lesions of pancreatic ductal adenocarcinoma and pancreatic injury. Oncogene 37, 1845–1856 (2018). https://doi.org/10.1038/s41388-017-0100-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-017-0100-4
This article is cited by
-
Molecular signaling in pancreatic ductal metaplasia: emerging biomarkers for detection and intervention of early pancreatic cancer
Cellular Oncology (2022)
-
MicroRNA-138-5p targets the NFIB-Snail1 axis to inhibit colorectal cancer cell migration and chemoresistance
Cancer Cell International (2020)
-
ERas regulates cell proliferation and epithelial–mesenchymal transition by affecting Erk/Akt signaling pathway in pancreatic cancer
Human Cell (2020)