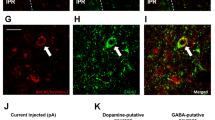
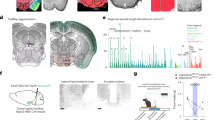
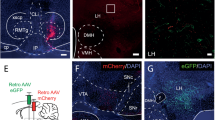
Abstract
The N-acyl phosphatidylethanolamine-specific phospholipase D (NAPE-PLD) catalyzes the production of N-acylethanolamines (NAEs), a family of endogenous bioactive lipids, which are involved in various biological processes ranging from neuronal functions to energy homeostasis and feeding behaviors. Reward-dependent behaviors depend on dopamine (DA) transmission between the ventral tegmental area (VTA) and the nucleus accumbens (NAc), which conveys reward-values and scales reinforced behaviors. However, whether and how NAPE-PLD may contribute to the regulation of feeding and reward-dependent behaviors has not yet been investigated. This biological question is of paramount importance since NAEs are altered in obesity and metabolic disorders. Here, we show that transcriptomic meta-analysis highlights a potential role for NAPE-PLD within the VTA→NAc circuit. Using brain-specific invalidation approaches, we report that the integrity of NAPE-PLD is required for the proper homeostasis of NAEs within the midbrain VTA and it affects food-reward behaviors. Moreover, region-specific knock-down of NAPE-PLD in the VTA enhanced food-reward seeking and reinforced behaviors, which were associated with increased in vivo DA release dynamics in response to both food- and non-food-related rewards together with heightened tropism towards food consumption. Furthermore, midbrain knock-down of NAPE-PLD, which increased energy expenditure and adapted nutrient partitioning, elicited a relative protection against high-fat diet-mediated body fat gain and obesity-associated metabolic features. In conclusion, these findings reveal a new key role of VTA NAPE-PLD in shaping DA-dependent events, feeding behaviors and energy homeostasis, thus providing new insights on the regulation of body metabolism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All the data generated have been included in the article. Datasets are available from the corresponding authors upon reasonable request.
References
Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–95.
Rossi MA, Stuber GD. Overlapping Brain Circuits for Homeostatic and Hedonic Feeding. Cell Metab. 2018;27:42–56.
de Araujo IE, Schatzker M, Small DM. Rethinking Food Reward. Annu Rev Psychol. 2020;71:139–64.
Berland C, Small DM, Luquet S, Gangarossa G. Dietary lipids as regulators of reward processes: multimodal integration matters. Trends Endocrinol Metab. 2021;32:693–705.
Mock ED, Gagestein B, van der Stelt M. Anandamide and other N-acylethanolamines: A class of signaling lipids with therapeutic opportunities. Prog Lipid Res. 2023;89:101194.
Hussain Z, Uyama T, Tsuboi K, Ueda N. Mammalian enzymes responsible for the biosynthesis of N -acylethanolamines. Biochim Biophys Acta. 2017;1862:1546–61.
Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–305.
Ahern GP. Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem. 2003;278:30429–34.
Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, et al. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93.
Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, et al. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharm. 2005;67:15–19.
Cristino L, Becker T, Di Marzo V. Endocannabinoids and energy homeostasis: an update. Biofactors. 2014;40:389–97.
Esposito G, Capoccia E, Turco F, Palumbo I, Lu J, Steardo A, et al. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut. 2014;63:1300–12.
Hansen HS, Vana V. Non-endocannabinoid N-acylethanolamines and 2-monoacylglycerols in the intestine. Br J Pharm. 2019;176:1443–54.
Rodriguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J, et al. An anorexic lipid mediator regulated by feeding. Nature. 2001;414:209–12.
Terrazzino S, Berto F, Dalle Carbonare M, Fabris M, Guiotto A, et al. Stearoylethanolamide exerts anorexic effects in mice via down-regulation of liver stearoyl-coenzyme A desaturase-1 mRNA expression. FASEB J. 2004;18:1580–2.
DiPatrizio NV. Endocannabinoids in the Gut. Cannabis Cannabinoid Res. 2016;1:67–77.
Fu J, Kim J, Oveisi F, Astarita G, Piomelli D. Targeted enhancement of oleoylethanolamide production in proximal small intestine induces across-meal satiety in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R45–50.
Everard A, Plovier H, Rastelli M, Van Hul M, de Wouters d’Oplinter A, Geurts L, et al. Intestinal epithelial N-acylphosphatidylethanolamine phospholipase D links dietary fat to metabolic adaptations in obesity and steatosis. Nat Commun. 2019;10:457.
Melis M, Pillolla G, Luchicchi A, Muntoni AL, Yasar S, Goldberg SR, et al. Endogenous Fatty Acid Ethanolamides Suppress Nicotine-Induced Activation of Mesolimbic Dopamine Neurons through Nuclear Receptors. J Neurosci. 2008;28:13985–94.
Palese F, Pontis S, Realini N, Piomelli D. A protective role for N-acylphosphatidylethanolamine phospholipase D in 6-OHDA-induced neurodegeneration. Sci Rep. 2019;9:15927.
Tevosian M, Todorov H, Lomazzo E, Bindila L, Ueda N, Bassetti D, et al. NAPE-PLD deletion in stress-TRAPed neurons results in an anxiogenic phenotype. Transl Psychiatry. 2023;13:152.
Morishita J, Okamoto Y, Tsuboi K, Ueno M, Sakamoto H, Maekawa N, et al. Regional distribution and age-dependent expression of N-acylphosphatidylethanolamine-hydrolyzing phospholipase D in rat brain. J Neurochem. 2005;94:753–62.
Egertova M, Simon GM, Cravatt BF, Elphick MR. Localization of N-acyl phosphatidylethanolamine phospholipase D (NAPE-PLD) expression in mouse brain: A new perspective on N-acylethanolamines as neural signaling molecules. J Comp Neurol. 2008;506:604–15.
Leishman E, Mackie K, Luquet S, Bradshaw HB. Lipidomics profile of a NAPE-PLD KO mouse provides evidence of a broader role of this enzyme in lipid metabolism in the brain. Biochim Biophys Acta. 2016;1861:491–500.
Mock ED, Mustafa M, Gunduz-Cinar O, Cinar R, Petrie GN, Kantae V, et al. Discovery of a NAPE-PLD inhibitor that modulates emotional behavior in mice. Nat Chem Biol. 2020;16:667–75.
Muccioli GG. Endocannabinoid biosynthesis and inactivation, from simple to complex. Drug Discov Today. 2010;15:474–83.
Leung D, Saghatelian A, Simon GM, Cravatt BF. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry. 2006;45:4720–6.
Wangensteen T, Akselsen H, Holmen J, Undlien D, Retterstøl L. A common haplotype in NAPEPLD is associated with severe obesity in a Norwegian population-based cohort (the HUNT study). Obesity. 2011;19:612–7.
Doris JM, Millar SA, Idris I, O’Sullivan SE. Genetic polymorphisms of the endocannabinoid system in obesity and diabetes. Diabetes Obes Metab. 2019;21:382–7.
Sun F, Zhou J, Dai B, Qian T, Zeng J, Li X, et al. Next-generation GRAB sensors for monitoring dopaminergic activity in vivo. Nat Methods. 2020;17:1156–66.
Berland C, Castel J, Terrasi R, Montalban E, Foppen E, Martin C, et al. Identification of an endocannabinoid gut-brain vagal mechanism controlling food reward and energy homeostasis. Mol Psychiatry. 2022;27:2340–54.
Berland C, Montalban E, Perrin E, Di Miceli M, Nakamura Y, Martinat M, et al. Circulating Triglycerides Gate Dopamine-Associated Behaviors through DRD2-Expressing Neurons. Cell Metab. 2020;31:773–e11.
Gangarossa G, Castell L, Castro L, Tarot P, Veyrunes F, Vincent P, et al. Contrasting patterns of ERK activation in the tail of the striatum in response to aversive and rewarding signals. J Neurochem. 2019;151:204–26.
Raboune S, Stuart JM, Leishman E, Takacs SM, Rhodes B, Basnet A, et al. Novel endogenous N-acyl amides activate TRPV1-4 receptors, BV-2 microglia, and are regulated in brain in an acute model of inflammation. Front Cell Neurosci. 2014;8:195.
Liu J, Wang L, Harvey-White J, Huang BX, Kim HY, Luquet S, et al. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology. 2008;54:1–7.
Geurts L, Everard A, Van Hul M, Essaghir A, Duparc T, Matamoros S, et al. Adipose tissue NAPE-PLD controls fat mass development by altering the browning process and gut microbiota. Nat Commun. 2015;6:6495.
Lallemand Y, Luria V, Haffner-Krausz R, Lonai P. Maternally expressed PGK-Cre transgene as a tool for early and uniform activation of the Cre site-specific recombinase. Transgenic Res. 1998;7:105–12.
Giusti SA, Vercelli CA, Vogl AM, Kolarz AW, Pino NS, Deussing JM, et al. Behavioral phenotyping of Nestin-Cre mice: implications for genetic mouse models of psychiatric disorders. J Psychiatr Res. 2014;55:87–95.
Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103.
Declercq J, Brouwers B, Pruniau VPEG, Stijnen P, de Faudeur G, Tuand K, et al. Metabolic and Behavioural Phenotypes in Nestin-Cre Mice Are Caused by Hypothalamic Expression of Human Growth Hormone. PLoS One. 2015;10:e0135502.
Tellez LA, Han W, Zhang X, Ferreira TL, Perez IO, Shammah-Lagnado SJ, et al. Separate circuitries encode the hedonic and nutritional values of sugar. Nat Neurosci. 2016;19:465–70.
Tellez LA, Medina S, Han W, Ferreira JG, Licona-Limon P, Ren X, et al. A gut lipid messenger links excess dietary fat to dopamine deficiency. Science. 2013;341:800–2.
Rastelli M, Van Hul M, Terrasi R, Lefort C, Regnier M, Beiroa D, et al. Intestinal NAPE-PLD contributes to short-term regulation of food intake via gut-to-brain axis. Am J Physiol Endocrinol Metab. 2020;319:E647–E657.
Morales I, Berridge KC. ‘Liking’ and ‘wanting’ in eating and food reward: Brain mechanisms and clinical implications. Physiol Behav. 2020;227:113152.
Savell KE, Tuscher JJ, Zipperly ME, Duke CG, Phillips RA, Bauman AJ, et al. A dopamine-induced gene expression signature regulates neuronal function and cocaine response. Sci Adv. 2020;6:eaba4221.
Phillips RA, Tuscher JJ, Black SL, Andraka E, Fitzgerald ND, Ianov L, et al. An atlas of transcriptionally defined cell populations in the rat ventral tegmental area. Cell Rep. 2022;39:110616.
Brichta L, Shin W, Jackson-Lewis V, Blesa J, Yap EL, Walker Z, et al. Identification of neurodegenerative factors using translatome-regulatory network analysis. Nat Neurosci. 2015;18:1325–33.
Aguila J, Cheng S, Kee N, Cao M, Wang M, Deng Q, et al. Spatial RNA Sequencing Identifies Robust Markers of Vulnerable and Resistant Human Midbrain Dopamine Neurons and Their Expression in Parkinson’s Disease. Front Mol Neurosci. 2021;14:699562.
Montalban E, Walle R, Castel J, Ansoult A, Hassouna R, Foppen E, et al. The addiction-susceptibility TaqIA/Ankyrin repeat and kinase domain containing 1 kinase (ANKK1) controls reward and metabolism through dopamine receptor type 2 (D2R)-expressing neurons. Biol Psychiatry. 2023;94:424–36.
Woodworth HL, Batchelor HM, Beekly BG, Bugescu R, Brown JA, Kurt G, et al. Neurotensin Receptor-1 Identifies a Subset of Ventral Tegmental Dopamine Neurons that Coordinates Energy Balance. Cell Rep. 2017;20:1881–92.
Fernandes MFA, Matthys D, Hryhorczuk C, Sharma S, Mogra S, Alquier T, et al. Leptin Suppresses the Rewarding Effects of Running via STAT3 Signaling in Dopamine Neurons. Cell Metab. 2015;22:741–9.
Medrano M-C, Hurel I, Mesguich E, Redon B, Stevens C, Georges F, et al. Exercise craving potentiates excitatory inputs to ventral tegmental area dopaminergic neurons. Addict Biol. 2021;26:e12967.
Muguruza C, Redon B, Fois GR, Hurel I, Scocard A, Nguyen C, et al. The motivation for exercise over palatable food is dictated by cannabinoid type-1 receptors. JCI Insight. 2019;4:e126190.
Dubreucq S, Durand A, Matias I, Bénard G, Richard E, Soria-Gomez E, et al. Ventral tegmental area cannabinoid type-1 receptors control voluntary exercise performance. Biol Psychiatry. 2013;73:895–903.
Lunerti V, Li H, Benvenuti F, Shen Q, Domi A, Soverchia L, et al. The multitarget FAAH inhibitor/D3 partial agonist ARN15381 decreases nicotine self-administration in male rats. Eur J Pharmacol. 2022;928:175088.
Melis M, Scheggi S, Carta G, Madeddu C, Lecca S, Luchicchi A, et al. PPARα regulates cholinergic-driven activity of midbrain dopamine neurons via a novel mechanism involving α7 nicotinic acetylcholine receptors. J Neurosci. 2013;33:6203–11.
Melis M, Carta S, Fattore L, Tolu S, Yasar S, Goldberg SR, et al. Peroxisome proliferator-activated receptors-alpha modulate dopamine cell activity through nicotinic receptors. Biol Psychiatry. 2010;68:256–64.
Hempel B, Crissman M, Pari S, Klein B, Bi G-H, Alton H, et al. PPARα and PPARγ are expressed in midbrain dopamine neurons and modulate dopamine- and cannabinoid-mediated behavior in mice. Mol Psychiatry. 2023. https://doi.org/10.1038/s41380-023-02182-0.
Guillaumin MCC, Viskaitis P, Bracey E, Burdakov D, Peleg-Raibstein D. Disentangling the role of NAc D1 and D2 cells in hedonic eating. Mol Psychiatry. 2023;28:3531–47.
Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95:S119–128.
Tsai H-C, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–4.
Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–73.
Kreitzer AC, Regehr WG. Retrograde signaling by endocannabinoids. Curr Opin Neurobiol. 2002;12:324–30.
Nyilas R, Dudok B, Urbán GM, Mackie K, Watanabe M, Cravatt BF, et al. Enzymatic machinery for endocannabinoid biosynthesis associated with calcium stores in glutamatergic axon terminals. J Neurosci. 2008;28:1058–63.
Pupe S, Wallén-Mackenzie Å. Cre-driven optogenetics in the heterogeneous genetic panorama of the VTA. Trends Neurosci. 2015;38:375–86.
Morales M, Margolis EB. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017;18:73–85.
Wang H-L, Qi J, Zhang S, Wang H, Morales M. Rewarding Effects of Optical Stimulation of Ventral Tegmental Area Glutamatergic Neurons. J Neurosci. 2015;35:15948–54.
Root DH, Barker DJ, Estrin DJ, Miranda-Barrientos JA, Liu B, Zhang S, et al. Distinct Signaling by Ventral Tegmental Area Glutamate, GABA, and Combinatorial Glutamate-GABA Neurons in Motivated Behavior. Cell Rep. 2020;32:108094.
Zell V, Steinkellner T, Hollon NG, Warlow SM, Souter E, Faget L, et al. VTA Glutamate Neuron Activity Drives Positive Reinforcement Absent Dopamine Co-release. Neuron. 2020;107:864–e4.
O’Connor EC, Kremer Y, Lefort S, Harada M, Pascoli V, Rohner C, et al. Accumbal D1R Neurons Projecting to Lateral Hypothalamus Authorize Feeding. Neuron. 2015;88:553–64.
Diepenbroek C, Rijnsburger M, van Irsen AAS, Eggels L, Kisner A, Foppen E, et al. Dopamine in the nucleus accumbens shell controls systemic glucose metabolism via the lateral hypothalamus and hepatic vagal innervation in rodents. Metabolism. 2023;150:155696.
Boekhoudt L, Omrani A, Luijendijk MCM, Wolterink-Donselaar IG, Wijbrans EC, van der Plasse G, et al. Chemogenetic activation of dopamine neurons in the ventral tegmental area, but not substantia nigra, induces hyperactivity in rats. Eur Neuropsychopharmacol. 2016;26:1784–93.
Perez-Bonilla P, Santiago-Colon K, Matasovsky J, Ramirez-Virella J, Khan R, Garver H, et al. Activation of ventral tegmental area neurotensin Receptor-1 neurons promotes weight loss. Neuropharmacology. 2021;195:108639.
Mietlicki-Baase EG, Reiner DJ, Cone JJ, Olivos DR, McGrath LE, Zimmer DJ, et al. Amylin modulates the mesolimbic dopamine system to control energy balance. Neuropsychopharmacology. 2015;40:372–85.
Fanelli F, Mezzullo M, Repaci A, Belluomo I, Ibarra Gasparini D, Di Dalmazi G, et al. Profiling plasma N-Acylethanolamine levels and their ratios as a biomarker of obesity and dysmetabolism. Mol Metab. 2018;14:82–94.
Jumpertz R, Guijarro A, Pratley RE, Piomelli D, Krakoff J. Central and peripheral endocannabinoids and cognate acylethanolamides in humans: association with race, adiposity, and energy expenditure. J Clin Endocrinol Metab. 2011;96:787–91.
Mazier W, Saucisse N, Gatta-Cherifi B, Cota D. The Endocannabinoid System: Pivotal Orchestrator of Obesity and Metabolic Disease. Trends Endocrinol Metab. 2015;26:524–37.
Acknowledgements
We thank Olja Kacanski for administrative support, Isabelle Le Parco, Aurélie Djemat, Daniel Quintas, Magguy Boa, Ludovic Maingault, Angélique Dauvin and Florianne Michel for animals’ care and genotyping. We thank Dr. Etienne Doumazane for clustering analyses and Dr. Sylvie Robin for the gift of Villin-CreERT2 mice. We acknowledge the technical platform Functional and Physiological Exploration platform (FPE) of the Université Paris Cité, CNRS, Unité de Biologie Fonctionnelle et Adaptative, and the animal core facility “Buffon” of the Université Paris Cité/Institut Jacques Monod.
Funding
This work was supported by the Nutricia Research Foundation (#2022-E7), Agence Nationale de la Recherche (ANR-21-CE14-0021-01, ANR-23-CE14-0014-02, ANR-19-CE37-0020-02), Fondation pour la Recherche Médicale (Équipe FRM #EQU202003010155), Fédération pour la Recherche sur le Cerveau and Association France Parkinson, Institut universtaire de France, the Modern Diet and Physiology Research Center (MDPRC), Université Paris Cité, US National Institutes of Health (DA047858), and CNRS. GL is supported by a China Scholarship Council (CSC) fellowship. OO is supported by an FRM fellowship. PDC is recipient of grants from FNRS (FRFS-WELBIO: WELBIO-CR-2022A-02, EOS: program no. 40007505) and La Caixa (NeuroGut). AE is research associate at FNRS and the recipient of grants from FNRS (FRFS-WELBIO: WELBIO-CR-2019S-03R, FNRS: J.0075.22).
Author information
Authors and Affiliations
Contributions
Conceptualization: GG and SL; Investigation: JC, GL, OO, AE, EL, HB, KM, PDC, SL, GG; Formal Data Analysis: JC, SL, GG, HB, KM, AE; Resources: GG, SL, PDC; Funding acquisition: GG, SL, PDC; Supervision: GG and SL; Writing – original draft: GG and SL; Writing – review & editing: all authors.
Corresponding authors
Ethics declarations
Competing interests
PDC and AE are inventors on patent applications dealing with the use of specific bacteria and components in the treatment of different diseases. PDC was co-founder of The Akkermansia Company SA and Enterosys. The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Castel, J., Li, G., Onimus, O. et al. NAPE-PLD in the ventral tegmental area regulates reward events, feeding and energy homeostasis. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-024-02427-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-024-02427-6