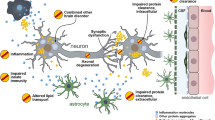
Abstract
Alzheimer’s disease (AD) is currently constrained by limited clinical treatment options. The initial pathophysiological event, which can be traced back to decades before the clinical symptoms become apparent, involves the excessive accumulation of amyloid-beta (Aβ), a peptide comprised of 40–42 amino acids, in extraneuronal plaques within the brain. Biochemical and histological studies have shown that overaccumulation of Aβ instigates an aberrant escalation in the phosphorylation and secretion of tau, a microtubule-binding axonal protein. The accumulation of hyperphosphorylated tau into intraneuronal neurofibrillary tangles is in turn correlated with microglial dysfunction and reactive astrocytosis, culminating in synaptic dysfunction and neurodegeneration. As neurodegeneration progresses, it gives rise to mild clinical symptoms of AD, which may eventually evolve into overt dementia. Synaptic loss in AD may develop even before tau alteration and in response to possible elevations in soluble oligomeric forms of Aβ associated with early AD. These findings largely rely on post-mortem autopsy examinations, which typically involve a limited number of patients. Over the past decade, a range of fluid biomarkers such as neurogranin, α-synuclein, visinin-like protein 1 (VILIP-1), neuronal pentraxin 2, and β-synuclein, along with positron emission tomography (PET) markers like synaptic vesicle glycoprotein 2A, have been developed. These advancements have facilitated the exploration of how synaptic markers in AD patients correlate with cognitive impairment. However, fluid biomarkers indicating synaptic loss have only been validated in cerebrospinal fluid (CSF), not in plasma, with the exception of VILIP-1. The most promising PET radiotracer, [11C]UCB-J, currently faces significant challenges hindering its widespread clinical use, primarily due to the necessity of a cyclotron. As such, additional research geared toward the exploration of synaptic pathology biomarkers is crucial. This will not only enable their extensive clinical application, but also refine the optimization process of AD pharmacological trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Dubois B, Hampel H, Feldman HH, Scheltens P, Aisen P, Andrieu S, et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323.
Gauthier S, Webster C, Servaes S, Morais JA, Rosa-Neto P. World Alzheimer Report 2022: life after diagnosis: navigating treatment, care and support. London: Alzheimer’s Disease International; 2022.
Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–91.
Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–80.
Blennow K, Bogdanovic N, Alafuzoff I, Ekman R, Davidsson P. Synaptic pathology in Alzheimer’s disease: relation to severity of dementia, but not to senile plaques, neurofibrillary tangles, or the ApoE4 allele. J Neural Transm (Vienna). 1996;103:603–18.
D’Amelio M, Nisticò R. Editorial Thematic Issue: Targeting synaptic dysfunction and neural connectivity in neurological and psychiatric disorders. Curr Pharm Des. 2013;19:6391–2.
Mango D, Saidi A, Cisale GY, Feligioni M, Corbo M, Nisticò R. Targeting synaptic plasticity in experimental models of Alzheimer’s disease. Front Pharm. 2019;10:778.
Aslam MM, Fan K-H, Lawrence E, Bedison MA, Snitz BE, DeKosky ST, et al. Genome-wide analysis identifies novel loci influencing plasma apolipoprotein E concentration and Alzheimer’s disease risk. Mol Psychiatry. 2023. https://doi.org/10.1038/s41380-023-02170-4.
Mimura Y, Nishida H, Nakajima S, Tsugawa S, Morita S, Yoshida K, et al. Neurophysiological biomarkers using transcranial magnetic stimulation in Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2021;121:47–59.
Mercerón-Martínez D, Ibaceta-González C, Salazar C, Almaguer-Melian W, Bergado-Rosado JA, Palacios AG. Alzheimer’s disease, neural plasticity, and functional recovery. J Alzheimers Dis. 2021;82:S37–S50.
Hefter D, Ludewig S, Draguhn A, Korte M. Amyloid, APP, and electrical activity of the brain. Neuroscientist. 2020;26:231–51.
Korte M, Herrmann U, Zhang X, Draguhn A. The role of APP and APLP for synaptic transmission, plasticity, and network function: lessons from genetic mouse models. Exp Brain Res. 2012;217:435–40.
Rowan MJ, Klyubin I, Cullen WK, Anwyl R. Synaptic plasticity in animal models of early Alzheimer’s disease. Philos Trans R Soc Lond B Biol Sci. 2003;358:821–8.
Motta C, Assogna M, Bonomi CG, Di Lorenzo F, Nuccetelli M, Mercuri NB, et al. Interplay between the catecholaminergic enzymatic axis and neurodegeneration/neuroinflammation processes in the Alzheimer’s disease continuum. Eur J Neurol. 2023;30:839–48.
Henjum K, Watne LO, Godang K, Halaas NB, Eldholm RS, Blennow K, et al. Cerebrospinal fluid catecholamines in Alzheimer’s disease patients with and without biological disease. Transl Psychiatry. 2022;12:151.
Nobili A, Latagliata EC, Viscomi MT, Cavallucci V, Cutuli D, Giacovazzo G, et al. Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer’s disease. Nat Commun. 2017;8:14727.
Sala A, Caminiti SP, Presotto L, Pilotto A, Liguori C, Chiaravalloti A, et al. In vivo human molecular neuroimaging of dopaminergic vulnerability along the Alzheimer’s disease phases. Alzheimers Res Ther. 2021;13:187.
Serra L, D’Amelio M, Esposito S, Di Domenico C, Koch G, Marra C, et al. Ventral tegmental area disconnection contributes two years early to correctly classify patients converted to Alzheimer’s disease: implications for treatment. J Alzheimers Dis. 2021;82:985–1000.
Serra L, D’Amelio M, Di Domenico C, Dipasquale O, Marra C, Mercuri NB, et al. In vivo mapping of brainstem nuclei functional connectivity disruption in Alzheimer’s disease. Neurobiol Aging. 2018;72:72–82.
Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, Amatniek J, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011;7:532–9.
Zhang H, Jiang X, Ma L, Wei W, Li Z, Chang S, et al. Role of Aβ in Alzheimer’s-related synaptic dysfunction. Front Cell Dev Biol. 2022;10:964075.
Brito-Moreira J, Lourenco MV, Oliveira MM, Ribeiro FC, Ledo JH, Diniz LP, et al. Interaction of amyloid-β (Aβ) oligomers with neurexin 2α and neuroligin 1 mediates synapse damage and memory loss in mice. J Biol Chem. 2017;292:7327–37.
Hartl D, Schuldt V, Forler S, Zabel C, Klose J, Rohe M. Presymptomatic alterations in energy metabolism and oxidative stress in the APP23 mouse model of Alzheimer disease. J Proteome Res. 2012;11:3295–304.
Zhang X-Q, Xu L, Yang S-Y, Hu L-B, Dong F-Y, Sun B-G, et al. Reduced synaptic transmission and intrinsic excitability of a subtype of pyramidal neurons in the medial prefrontal cortex in a mouse model of Alzheimer’s disease. J Alzheimers Dis. 2021;84:129–40.
Cassano T, Serviddio G, Gaetani S, Romano A, Dipasquale P, Cianci S, et al. Glutamatergic alterations and mitochondrial impairment in a murine model of Alzheimer disease. Neurobiol Aging. 2012;33:1121.e1–12.
Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–9.
Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, et al. β-Amyloid peptides enhance α-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer’s disease and Parkinson’s disease. Proc Natl Acad Sci USA. 2001;98:12245–50.
Spencer B, Desplats PA, Overk CR, Valera-Martin E, Rissman RA, Wu C, et al. Reducing endogenous α-synuclein mitigates the degeneration of selective neuronal populations in an Alzheimer’s disease transgenic mouse model. J Neurosci. 2016;36:7971–84.
Chandra S, Fornai F, Kwon H-B, Yazdani U, Atasoy D, Liu X, et al. Double-knockout mice for α- and β-synucleins: effect on synaptic functions. Proc Natl Acad Sci USA. 2004;101:14966–71.
Liu S, Fa M, Ninan I, Trinchese F, Dauer W, Arancio O. Alpha-Synuclein involvement in hippocampal synaptic plasticity: role of NO, cGMP, cGK and CaMKII. Eur J Neurosci. 2007;25:3583–96.
Fan Y, Limprasert P, Murray IVJ, Smith AC, Lee VM-Y, Trojanowski JQ, et al. β-synuclein modulates α-synuclein neurotoxicity by reducing α-synuclein protein expression. Hum Mol Genet. 2006;15:3002–11.
Lleó A, Núñez-Llaves R, Alcolea D, Chiva C, Balateu-Paños D, Colom-Cadena M, et al. Changes in synaptic proteins precede neurodegeneration markers in preclinical Alzheimer’s disease cerebrospinal fluid. Mol Cell Proteom. 2019;18:546–60.
Zhong L, Gerges NZ. Neurogranin targets calmodulin and lowers the threshold for the induction of long-term potentiation. PLoS ONE. 2012;7:e41275.
Petersen A, Gerges NZ. Neurogranin regulates CaM dynamics at dendritic spines. Sci Rep. 2015;5:11135.
Pak JH, Huang FL, Li J, Balschun D, Reymann KG, Chiang C, et al. Involvement of neurogranin in the modulation of calcium/calmodulin-dependent protein kinase II, synaptic plasticity, and spatial learning: a study with knockout mice. Proc Natl Acad Sci USA. 2000;97:11232–7.
Huang K-P, Huang FL, Jäger T, Li J, Reymann KG, Balschun D. Neurogranin/RC3 enhances long-term potentiation and learning by promoting calcium-mediated signaling. J Neurosci. 2004;24:10660–9.
Braunewell KH. The visinin-like proteins VILIP-1 and VILIP-3 in Alzheimer’s disease—old wine in new bottles. Front Mol Neurosci. 2012;5:20.
DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol. 1990;27:457–64.
The Synaptic Health Endpoints Working Group, Colom-Cadena M, Spires-Jones T, Zetterberg H, Blennow K, Caggiano A, et al. The clinical promise of biomarkers of synapse damage or loss in Alzheimer’s disease. Alzheimers Res Ther. 2020;12:21.
Camporesi E, Nilsson J, Brinkmalm A, Becker B, Ashton NJ, Blennow K, et al. Fluid biomarkers for synaptic dysfunction and loss. Biomark Insights. 2020;15:117727192095031.
Wang Z-Y, Han Z-M, Liu Q-F, Tang W, Ye K, Yao Y-Y. Use of CSF α-synuclein in the differential diagnosis between Alzheimer’s disease and other neurodegenerative disorders. Int Psychogeriatr. 2015;27:1429–38.
Tu S, Okamoto S, Lipton SA, Xu H. Oligomeric Aβ-induced synaptic dysfunction in Alzheimer’s disease. Mol Neurodegener. 2014;9:48.
Guerrero-Muñoz MJ, Gerson J, Castillo-Carranza DL. Tau oligomers: the toxic player at synapses in Alzheimer’s disease. Front Cell Neurosci. 2015;9:464.
Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–75.
Cline EN, Bicca MA, Viola KL, Klein WL. The amyloid-β oligomer hypothesis: beginning of the third decade. J Alzheimers Dis. 2018;64:S567–S610.
Colom-Cadena M, Davies C, Sirisi S, Lee J-E, Simzer EM, Tzioras M, et al. Synaptic oligomeric tau in Alzheimer’s disease — a potential culprit in the spread of tau pathology through the brain. Neuron. 2023;111:2170–83.e6. S0896627323003057.
Puzzo D, Piacentini R, Fá M, Gulisano W, Li Puma DD, Staniszewski A, et al. LTP and memory impairment caused by extracellular Aβ and Tau oligomers is APP-dependent. eLife. 2017;6:e26991.
Jadhav S, Cubinkova V, Zimova I, Brezovakova V, Madari A, Cigankova V, et al. Tau-mediated synaptic damage in Alzheimer’s disease. Transl Neurosci. 2015;6:214–26.
Cardozo PL, De Lima IBQ, Maciel EMA, Silva NC, Dobransky T, Ribeiro FM. Synaptic elimination in neurological disorders. Curr Neuropharmacol. 2019;17:1071–95.
Griffiths J, Grant SGN. Synapse pathology in Alzheimer’s disease. Semin Cell Dev Biol. 2023;139:13–23.
Galasko D, Xiao M, Xu D, Smirnov D, Salmon DP, Dewit N, et al. Synaptic biomarkers in CSF aid in diagnosis, correlate with cognition and predict progression in MCI and Alzheimer’s disease. Alzheimers Dement Transl Res Clin Inter. 2019;5:871–82.
Milà-Alomà M, Brinkmalm A, Ashton NJ, Kvartsberg H, Shekari M, Operto G, et al. CSF synaptic biomarkers in the preclinical stage of Alzheimer disease and their association with MRI and PET: a cross-sectional study. Neurology. 2021;97:e2065–78.
Represa A, Deloulme J, Sensenbrenner M, Ben-Ari Y, Baudier J. Neurogranin: immunocytochemical localization of a brain-specific protein kinase C substrate. J Neurosci. 1990;10:3782–92.
Gerendasy DD, Gregor Sutcliffe J. RC3/neurogranin, a postsynaptic calpacitin for setting the response threshold to calcium influxes. Mol Neurobiol. 1997;15:131–63.
Xia Z, Storm DR. The role of calmodulin as a signal integrator for synaptic plasticity. Nat Rev Neurosci. 2005;6:267–76.
Hayashi Y. Long-term potentiation: two pathways meet at neurogranin. EMBO J. 2009;28:2859–60.
Thorsell A, Bjerke M, Gobom J, Brunhage E, Vanmechelen E, Andreasen N, et al. Neurogranin in cerebrospinal fluid as a marker of synaptic degeneration in Alzheimer’s disease. Brain Res. 2010;1362:13–22.
Kvartsberg H, Duits FH, Ingelsson M, Andreasen N, Öhrfelt A, Andersson K, et al. Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer’s disease. Alzheimers Dement. 2015;11:1180–90.
Kester MI, Teunissen CE, Crimmins DL, Herries EM, Ladenson JH, Scheltens P, et al. Neurogranin as a cerebrospinal fluid biomarker for synaptic loss in symptomatic Alzheimer disease. JAMA Neurol. 2015;72:1275.
Portelius E, Zetterberg H, Skillbäck T, Törnqvist U, Andreasson U, Trojanowski JQ, et al. Cerebrospinal fluid neurogranin: relation to cognition and neurodegeneration in Alzheimer’s disease. Brain. 2015;138:3373–85.
Kvartsberg H, Portelius E, Andreasson U, Brinkmalm G, Hellwig K, Lelental N, et al. Characterization of the postsynaptic protein neurogranin in paired cerebrospinal fluid and plasma samples from Alzheimer’s disease patients and healthy controls. Alzheimers Res Ther. 2015;7:40.
De Vos A, Jacobs D, Struyfs H, Fransen E, Andersson K, Portelius E, et al. C‐terminal neurogranin is increased in cerebrospinal fluid but unchanged in plasma in Alzheimer’s disease. Alzheimers Dement. 2015;11:1461–9.
Tarawneh R, D’Angelo G, Crimmins D, Herries E, Griest T, Fagan AM, et al. Diagnostic and prognostic utility of the synaptic marker neurogranin in Alzheimer disease. JAMA Neurol. 2016;73:561.
Sanfilippo C, Forlenza O, Zetterberg H, Blennow K. Increased neurogranin concentrations in cerebrospinal fluid of Alzheimer’s disease and in mild cognitive impairment due to AD. J Neural Transm. 2016;123:1443–7.
For the Alzheimer Precision Medicine Initiative (APMI), Lista S, Toschi N, Baldacci F, Zetterberg H, Blennow K, et al. Cerebrospinal fluid neurogranin as a biomarker of neurodegenerative diseases: a cross-sectional study. J Alzheimers Dis. 2017;59:1327–34.
Sutphen CL, McCue L, Herries EM, Xiong C, Ladenson JH, Holtzman DM, et al. Longitudinal decreases in multiple cerebrospinal fluid biomarkers of neuronal injury in symptomatic late onset Alzheimer’s disease. Alzheimers Dement. 2018;14:869–79.
Blennow K, Diaz-Lucena D, Zetterberg H, Villar-Pique A, Karch A, Vidal E, et al. CSF neurogranin as a neuronal damage marker in CJD: a comparative study with AD. J Neurol Neurosurg Psychiatry. 2019;90:846–53.
Wong YC, Krainc D. α-Synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med. 2017;23:1–13.
Colom-Cadena M, Pegueroles J, Herrmann AG, Henstridge CM, Muñoz L, Querol-Vilaseca M, et al. Synaptic phosphorylated α-synuclein in dementia with Lewy bodies. Brain. 2017;140:3204–14.
Goedert M. Alzheimer’s and Parkinson’s diseases: the prion concept in relation to assembled Aβ, tau, and α-synuclein. Science. 2015;349:1255555.
Slaets S, Vanmechelen E, Le Bastard N, Decraemer H, Vandijck M, Martin J, et al. Increased CSF α‐synuclein levels in Alzheimer’s disease: correlation with tau levels. Alzheimers Dement. 2014;10:S290–8.
Hansson O, Hall S, Öhrfelt A, Zetterberg H, Blennow K, Minthon L, et al. Levels of cerebrospinal fluid α-synuclein oligomers are increased in Parkinson’s disease with dementia and dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimers Res Ther. 2014;6:25.
Mattsson N, Insel P, Tosun D, Zhang J, Jack Jr CR, Galasko D, et al. Effects of baseline CSF α-synuclein on regional brain atrophy rates in healthy elders, mild cognitive impairment and Alzheimer’s disease. PLoS ONE. 2013;8:e85443.
Vergallo A, Bun R, Toschi N, Baldacci F, Zetterberg H, Blennow K, et al. Association of cerebrospinal fluid α‐synuclein with total and phospho‐tau 181 protein concentrations and brain amyloid load in cognitively normal subjective memory complainers stratified by Alzheimer’s disease biomarkers. Alzheimers Dement. 2018;14:1623–31.
Laske C, Fallgatter AJ, Stransky E, Hagen K, Berg D, Maetzler W. Decreased α-synuclein serum levels in patients with lewy body dementia compared to Alzheimer’s disease patients and control subjects. Dement Geriatr Cogn Disord. 2011;31:413–6.
Baldacci F, Daniele S, Piccarducci R, Giampietri L, Pietrobono D, Giorgi FS, et al. Potential diagnostic value of red blood cells α-synuclein heteroaggregates in Alzheimer’s disease. Mol Neurobiol. 2019;56:6451–9.
Daniele S, Baldacci F, Piccarducci R, Palermo G, Giampietri L, Manca ML, et al. α-Synuclein heteromers in red blood cells of Alzheimer’s disease and Lewy body dementia patients. J Alzheimers Dis. 2021;80:885–93.
Oeckl P, Metzger F, Nagl M, Von Arnim CAF, Halbgebauer S, Steinacker P, et al. Alpha-, beta-, and gamma-synuclein quantification in cerebrospinal fluid by multiple reaction monitoring reveals increased concentrations in Alzheimer′s and Creutzfeldt-Jakob disease but no alteration in synucleinopathies. Mol Cell Proteom. 2016;15:3126–38.
Mohaupt P, Pons M-L, Vialaret J, Delaby C, Hirtz C, Lehmann S. β-Synuclein as a candidate blood biomarker for synaptic degeneration in Alzheimer’s disease. Alzheimers Res Ther. 2022;14:179.
Oeckl P, Bluma M, Bucci M, Halbgebauer S, Chiotis K, Sandebring‐Matton A, et al. Blood β‐synuclein is related to amyloid PET positivity in memory clinic patients. Alzheimers Dement. 2023;19:4896–907. alz.13046.
Oeckl P, Janelidze S, Halbgebauer S, Stomrud E, Palmqvist S, Otto M, et al. Higher plasma β‐synuclein indicates early synaptic degeneration in Alzheimer’s disease. Alzheimers Dement. 2023;19:5095–102. alz.13103
Groblewska M, Muszyński P, Wojtulewska-Supron A, Kulczyńska-Przybik A, Mroczko B. The role of visinin-like protein-1 in the pathophysiology of Alzheimer’s disease. J Alzheimers Dis. 2015;47:17–32.
Braunewell K-H, Riederer P, Spilker C, Gundelfinger ED, Bogerts B, Bernstein H-G. Abnormal localization of two neuronal calcium sensor proteins, visinin-like proteins (VILIPs)-1 and -3, in neocortical brain areas of Alzheimer disease patients. Dement Geriatr Cogn Disord. 2001;12:110–6.
Lee J-M, Blennow K, Andreasen N, Laterza O, Modur V, Olander J, et al. The brain injury biomarker VLP-1 is increased in the cerebrospinal fluid of Alzheimer disease patients. Clin Chem. 2008;54:1617–23.
Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–84.
Tarawneh R, D’Angelo G, Macy E, Xiong C, Carter D, Cairns NJ, et al. Visinin-like protein-1: diagnostic and prognostic biomarker in Alzheimer disease. Ann Neurol. 2011;70:274–85.
Luo X, Hou L, Shi H, Zhong X, Zhang Y, Zheng D, et al. CSF levels of the neuronal injury biomarker visinin-like protein-1 in Alzheimer’s disease and dementia with Lewy bodies. J Neurochem. 2013;127:681–90.
Babić Leko M, Borovečki F, Dejanović N, Hof PR, Šimić G. Predictive value of cerebrospinal fluid visinin-like protein-1 levels for Alzheimer’s disease early detection and differential diagnosis in patients with mild cognitive impairment. J Alzheimers Dis. 2016;50:765–78.
Tarawneh R, Head D, Allison S, Buckles V, Fagan AM, Ladenson JH, et al. Cerebrospinal fluid markers of neurodegeneration and rates of brain atrophy in early Alzheimer disease. JAMA Neurol. 2015;72:656.
Tarawneh R, Lee J-M, Ladenson JH, Morris JC, Holtzman DM. CSF VILIP-1 predicts rates of cognitive decline in early Alzheimer disease. Neurology. 2012;78:709–19.
Fagan AM, Xiong C, Jasielec MS, Bateman RJ, Goate AM, Benzinger TLS, et al. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Sci Transl Med. 2014;6:226ra30.
Mavroudis IA, Petridis F, Chatzikonstantinou S, Karantali E, Kazis D. A meta-analysis on the levels of VILIP-1 in the CSF of Alzheimer’s disease compared to normal controls and other neurodegenerative conditions. Aging Clin Exp Res. 2021;33:265–72.
Öhrfelt A, Brinkmalm A, Dumurgier J, Brinkmalm G, Hansson O, Zetterberg H, et al. The pre-synaptic vesicle protein synaptotagmin is a novel biomarker for Alzheimer’s disease. Alzheimers Res Ther. 2016;8:41.
Antonucci F, Corradini I, Fossati G, Tomasoni R, Menna E, Matteoli M. SNAP-25, a known presynaptic protein with emerging postsynaptic functions. Front Synaptic Neurosci. 2016;8:7.
Brinkmalm A, Brinkmalm G, Honer WG, Frölich L, Hausner L, Minthon L, et al. SNAP-25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer’s disease. Mol Neurodegener. 2014;9:53.
The Alzheimer’s Disease Neuroimaging Initiative, Zhang H, Therriault J, Kang MS, Ng KP, Pascoal TA, et al. Cerebrospinal fluid synaptosomal-associated protein 25 is a key player in synaptic degeneration in mild cognitive impairment and Alzheimer’s disease. Alzheimers Res Ther. 2018;10:80.
Schindler SE, Li Y, Todd KW, Herries EM, Henson RL, Gray JD, et al. Emerging cerebrospinal fluid biomarkers in autosomal dominant Alzheimer’s disease. Alzheimers Dement. 2019;15:655–65.
Das S, Goossens J, Jacobs D, Dewit N, Pijnenburg YAL, In ‘T Veld SGJG, et al. Synaptic biomarkers in the cerebrospinal fluid associate differentially with classical neuronal biomarkers in patients with Alzheimer’s disease and frontotemporal dementia. Alzheimers Res Ther. 2023;15:62.
Rosskothen-Kuhl N, Illing R-B. Gap43 transcription modulation in the adult brain depends on sensory activity and synaptic cooperation. PLoS ONE. 2014;9:e92624.
Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91.
Sandelius Å, Portelius E, Källén Å, Zetterberg H, Rot U, Olsson B, et al. Elevated CSF GAP‐43 is Alzheimer’s disease specific and associated with tau and amyloid pathology. Alzheimers Dement. 2019;15:55–64.
Gómez De San José N, Massa F, Halbgebauer S, Oeckl P, Steinacker P, Otto M. Neuronal pentraxins as biomarkers of synaptic activity: from physiological functions to pathological changes in neurodegeneration. J Neural Transm. 2022;129:207–30.
Xiao M-F, Xu D, Craig MT, Pelkey KA, Chien C-C, Shi Y, et al. NPTX2 and cognitive dysfunction in Alzheimer’s Disease. eLife. 2017;6:e23798.
Belbin O, Xiao M-F, Xu D, Carmona-Iragui M, Pegueroles J, Benejam B, et al. Cerebrospinal fluid profile of NPTX2 supports role of Alzheimer’s disease-related inhibitory circuit dysfunction in adults with Down syndrome. Mol Neurodegener. 2020;15:46.
Soldan A, Oh S, Ryu T, Pettigrew C, Zhu Y, Moghekar A, et al. NPTX2 in cerebrospinal fluid predicts the progression from normal cognition to Mild Cognitive Impairment. Ann Neurol. 2023;94:620–31. ana.26725
Reiman EM. Fluorodeoxyglucose positron emission tomography: emerging roles in the evaluation of putative Alzheimer’s disease-modifying treatments. Neurobiol Aging. 2011;32:S44–7.
Zimmer ER, Parent MJ, Souza DG, Leuzy A, Lecrux C, Kim H-I, et al. [18F]FDG PET signal is driven by astroglial glutamate transport. Nat Neurosci. 2017;20:393–5.
Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET evaluation of cerebral metabolic decline in dementia: a potential outcome measure in Alzheimer’s disease treatment studies. Am J Psychiatry. 2002;159:738–45.
Rossi R, Arjmand S, Bærentzen SL, Gjedde A, Landau AM. Synaptic vesicle glycoprotein 2A: features and functions. Front Neurosci. 2022;16:864514.
Cawthorne C, Maguire P, Mercier J, Sciberras D, Serdons K, Bormans G, et al. Human biodistribution and dosimetry of [11C]-UCB-J, a PET radiotracer for imaging synaptic density. EJNMMI Phys. 2021;8:37.
Chen M-K, Mecca AP, Naganawa M, Finnema SJ, Toyonaga T, Lin S-F, et al. Assessing synaptic density in Alzheimer disease with synaptic vesicle glycoprotein 2A positron emission tomographic imaging. JAMA Neurol. 2018;75:1215–24.
Bastin C, Bahri MA, Meyer F, Manard M, Delhaye E, Plenevaux A, et al. In vivo imaging of synaptic loss in Alzheimer’s disease with [18F]UCB-H positron emission tomography. Eur J Nucl Med Mol Imaging. 2020;47:390–402.
Patel S, Knight A, Krause S, Teceno T, Tresse C, Li S, et al. Preclinical in vitro and in vivo characterization of synaptic vesicle 2A–targeting compounds amenable to F-18 labeling as potential PET radioligands for imaging of synapse integrity. Mol Imaging Biol. 2020;22:832–41.
Constantinescu CC, Tresse C, Zheng M, Gouasmat A, Carroll VM, Mistico L, et al. Development and in vivo preclinical imaging of fluorine-18-labeled synaptic vesicle protein 2A (SV2A) PET tracers. Mol Imaging Biol. 2019;21:509–18.
Scheff SW, Neltner JH, Nelson PT. Is synaptic loss a unique hallmark of Alzheimer’s disease? Biochem Pharm. 2014;88:517–28.
Vrillon A, Mouton-Liger F, Martinet M, Cognat E, Hourregue C, Dumurgier J, et al. Plasma neuregulin 1 as a synaptic biomarker in Alzheimer’s disease: a discovery cohort study. Alzheimers Res Ther. 2022;14:71.
Goetzl EJ, Kapogiannis D, Schwartz JB, Lobach IV, Goetzl L, Abner EL, et al. Decreased synaptic proteins in neuronal exosomes of frontotemporal dementia and Alzheimer’s disease. FASEB J. 2016;30:4141–8.
Goetzl EJ, Abner EL, Jicha GA, Kapogiannis D, Schwartz JB. Declining levels of functionally specialized synaptic proteins in plasma neuronal exosomes with progression of Alzheimer’s disease. FASEB J. 2018;32:888–93.
He M, Sun L, Cao W, Yin C, Sun W, Liu P, et al. Association between plasma exosome neurogranin and brain structure in patients with Alzheimer’s disease: a protocol study. BMJ Open. 2020;10:e036990.
Liu W, Lin H, He X, Chen L, Dai Y, Jia W, et al. Neurogranin as a cognitive biomarker in cerebrospinal fluid and blood exosomes for Alzheimer’s disease and mild cognitive impairment. Transl Psychiatry. 2020;10:125.
Nilsson J, Constantinescu J, Nellgård B, Jakobsson P, Brum WS, Gobom J, et al. Cerebrospinal fluid biomarkers of synaptic dysfunction are altered in Parkinson’s disease and related disorders. Mov Disord. 2023;38:267–77.
Sogorb-Esteve A, Nilsson J, Swift IJ, Heller C, Bocchetta M, Russell LL, et al. Differential impairment of cerebrospinal fluid synaptic biomarkers in the genetic forms of frontotemporal dementia. Alzheimers Res Ther. 2022;14:118.
Hurst C, Pugh DA, Abreha MH, Duong DM, Dammer EB, Bennett DA, et al. Integrated proteomics to understand the role of neuritin (NRN1) as a mediator of cognitive resilience to Alzheimer’s disease. Mol Cell Proteom. 2023;22:100542.
Johnson ECB, Bian S, Haque RU, Carter EK, Watson CM, Gordon BA, et al. Cerebrospinal fluid proteomics define the natural history of autosomal dominant Alzheimer’s disease. Nat Med. 2023;29:1979–88.
Vrahatis AG, Skolariki K, Krokidis MG, Lazaros K, Exarchos TP, Vlamos P. Revolutionizing the early detection of Alzheimer’s disease through non-invasive biomarkers: the role of artificial intelligence and deep learning. Sensors. 2023;23:4184.
Borchert RJ, Azevedo T, Badhwar A, Bernal J, Betts M, Bruffaerts R, et al. Artificial intelligence for diagnostic and prognostic neuroimaging in dementia: a systematic review. Alzheimers Dement. 2023:alz.13412.
Winchester LM, Harshfield EL, Shi L, Badhwar A, Khleifat AA, Clarke N, et al. Artificial intelligence for biomarker discovery in Alzheimer’s disease and dementia. Alzheimers Dement. 2023:alz.13390.
Rossini PM, Miraglia F, Alù F, Cotelli M, Ferreri F, Iorio RD, et al. Neurophysiological hallmarks of neurodegenerative cognitive decline: the study of brain connectivity as a biomarker of early dementia. J Pers Med. 2020;10:34.
Mandal PK, Shukla D. Brain metabolic, structural, and behavioral pattern learning for early predictive diagnosis of Alzheimer’s disease. J Alzheimers Dis. 2018;63:935–9.
Acknowledgements
Research by SL-O is funded by the Spanish Ministry of Science and Innovation (grant number FPU19/02117). HZ is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2022-01018 and #2019-02397), the European Union’s Horizon Europe research and innovation programme under grant agreement No 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C, and #ADSF-21-831377-C), the Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), the European Union Joint Programme – Neurodegenerative Disease Research (JPND2021-00694), the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre, and the UK Dementia Research Institute at UCL (UKDRI-1003).
Author information
Authors and Affiliations
Contributions
SL conceptualized the article, performed a critical review of the literature, wrote and revised the manuscript. AS-L and EE contributed to the writing and revision of the article and supervised artwork. NBM, AG, SL-O, JM-H, NM, CI, and FC supported the critical review of the literature, the writing, and/or the revision of the article. BPI and HZ contributed to the writing and revision of the article and supervised artwork. RN conceptualized the article, performed a critical review of the literature, wrote and revised the manuscript. All Authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
EE is the unique owner of 2E Science, a for-profit private scientific company. Neither EE nor 2E Science have any commercial interest or financial tie in relation with this article. BPI is an employee at Chiesi Farmaceutici. He is listed among the inventors of a number of Chiesi Farmaceutici’s patents of anti-Alzheimer drugs. HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). SL, AS-L, NBM, AG, SL-O, JM-H, NM, CI, FC, and RN declare that they have no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lista, S., Santos-Lozano, A., Emanuele, E. et al. Monitoring synaptic pathology in Alzheimer’s disease through fluid and PET imaging biomarkers: a comprehensive review and future perspectives. Mol Psychiatry (2024). https://doi.org/10.1038/s41380-023-02376-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-023-02376-6