Abstract
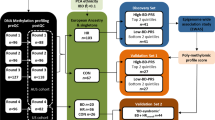
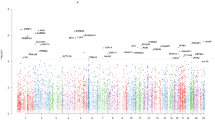
Anxiety Disorders (ANX) such as panic disorder, generalized anxiety disorder, and phobias, are highly prevalent conditions that are moderately heritable. Evidence suggests that DNA methylation may play a role, as it is involved in critical adaptations to changing environments. Applying an enrichment-based sequencing approach covering nearly 28 million autosomal CpG sites, we conducted a methylome-wide association study (MWAS) of lifetime ANX in 1132 participants (618 cases/514 controls) from the Netherlands Study of Depression and Anxiety. Using epigenomic deconvolution, we performed MWAS for the main cell types in blood: granulocytes, T-cells, B-cells and monocytes. Cell-type specific analyses identified 280 and 82 methylome-wide significant associations (q-value < 0.1) in monocytes and granulocytes, respectively. Our top finding in monocytes was located in ZNF823 on chromosome 19 (p = 1.38 × 10−10) previously associated with schizophrenia. We observed significant overlap (p < 1 × 10−06) with the same direction of effect in monocytes (210 sites), T-cells (135 sites), and B-cells (727 sites) between this Discovery MWAS signal and a comparable replication dataset from the Great Smoky Mountains Study (N = 433). Overlapping Discovery-Replication MWAS signal was enriched for findings from published GWAS of ANX, major depression, and post-traumatic stress disorder. In monocytes, two specific sites in the FZR1 gene showed significant replication after Bonferroni correction with an additional 15 nominally replicated sites in monocytes and 4 in T-cells. FZR1 regulates neurogenesis in the hippocampus, and its knockout leads to impairments in associative fear memory and long-term potentiation in mice. In the largest and most extensive methylome-wide study of ANX, we identified replicable methylation sites located in genes of potential relevance for brain mechanisms of psychiatric conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Full summary results are available upon request from the corresponding author. NESDA and GSMS data are available through an application and approval process through their corresponding institutions.
References
Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602.
Ask H, Cheesman R, Jami ES, Levey DF, Purves KL, Weber H. Genetic contributions to anxiety disorders: where we are and where we are heading. Psychol Med. 2021;51:2231–46.
Trubetskoy V, Pardinas AF, Qi T, Panagiotaropoulou G, Awasthi S, Bigdeli TB, et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature. 2022;604:502–8.
Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22:343–52.
Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–81.
Alameda L, Trotta G, Quigley H, Rodriguez V, Gadelrab R, Dwir D, et al. Can epigenetics shine a light on the biological pathways underlying major mental disorders? Psychol Med. 2022;52:1645–65.
Wiegand A, Kreifelts B, Munk MHJ, Geiselhart N, Ramadori KE, MacIsaac JL, et al. DNA methylation differences associated with social anxiety disorder and early life adversity. Transl Psychiatry. 2021;11:104.
Schiele MA, Gottschalk MG, Domschke K. The applied implications of epigenetics in anxiety, affective and stress-related disorders - A review and synthesis on psychosocial stress, psychotherapy and prevention. Clin Psychol Rev. 2020;77:101830.
Shimada-Sugimoto M, Otowa T, Miyagawa T, Umekage T, Kawamura Y, Bundo M, et al. Epigenome-wide association study of DNA methylation in panic disorder. Clin Epigenet. 2017;9:6.
Iurato S, Carrillo-Roa T, Arloth J, Czamara D, Diener-Holzl L, Lange J, et al. "DNA Methylation signatures in panic disorder". Transl Psychiatry. 2017;7:1287.
Ziegler C, Grundner-Culemann F, Schiele MA, Schlosser P, Kollert L, Mahr M, et al. The DNA methylome in panic disorder: a case-control and longitudinal psychotherapy-epigenetic study. Transl Psychiatry. 2019;9:314.
Ciuculete DM, Bostrom AE, Tuunainen AK, Sohrabi F, Kular L, Jagodic M, et al. Changes in methylation within the STK32B promoter are associated with an increased risk for generalized anxiety disorder in adolescents. J Psychiatr Res. 2018;102:44–51.
Emeny RT, Baumert J, Zannas AS, Kunze S, Wahl S, Iurato S, et al. Anxiety Associated Increased CpG Methylation in the Promoter of Asb1: A Translational Approach Evidenced by Epidemiological and Clinical Studies and a Murine Model. Neuropsychopharmacology 2018;43:342–53.
Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinforma. 2012;13:86.
Qi L, Teschendorff AE. Cell-type heterogeneity: Why we should adjust for it in epigenome and biomarker studies. Clin Epigenet. 2022;14:31.
Shen-Orr SS, Gaujoux R. Computational deconvolution: extracting cell type-specific information from heterogeneous samples. Curr Opin Immunol. 2013;25:571–8.
Chan RF, Shabalin AA, Xie LY, Adkins DE, Zhao M, Turecki G, et al. Enrichment methods provide a feasible approach to comprehensive and adequately powered investigations of the brain methylome. Nucleic Acids Res. 2017;45:e7.
Aberg KA, Dean B, Shabalin AA, Chan RF, Han LKM, Zhao M, et al. Methylome-wide association findings for major depressive disorder overlap in blood and brain and replicate in independent brain samples. Mol Psychiatry. 2020;25:1344–54.
Penninx BW, Beekman AT, Smit JH, Zitman FG, Nolen WA, Spinhoven P, et al. The Netherlands Study of Depression and Anxiety (NESDA): rationale, objectives and methods. Int J Methods Psychiatr Res. 2008;17:121–40.
Wittchen HU. Reliability and validity studies of the WHO–Composite International Diagnostic Interview (CIDI): a critical review. J Psychiatr Res. 1994;28:57–84.
Aberg KA, Chan RF, van den Oord E. MBD-seq - realities of a misunderstood method for high-quality methylome-wide association studies. Epigenetics 2020;15:431–8.
Aberg KA, Chan RF, Shabalin AA, Zhao M, Turecki G, Heine Staunstrup N, et al. A MBD-seq protocol for large-scale methylome-wide studies with (very) low amounts of DNA. Epigenetics. 2017;12:743–50.
Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–9.
Shabalin AA, Hattab MW, Clark SL, Chan RF, Kumar G, Aberg KA, et al. RaMWAS: Fast Methylome-Wide Association Study Pipeline for Enrichment Platforms. Bioinformatics. 2018;34:2283–5.
van den Oord EJ, Bukszar J, Rudolf G, Nerella S, McClay JL, Xie LY, et al. Estimation of CpG coverage in whole methylome next-generation sequencing studies. BMC Bioinforma. 2013;14:50.
Storey JD. The positive false discovery rate: A Bayesian interpretation and the q-value. Ann Stat. 2003;31:2013–35.
van den Oord EJ, Sullivan PF. False discoveries and models for gene discovery. Trends Genet. 2003;19:537–42.
Onuchic V, Hartmaier RJ, Boone DN, Samuels ML, Patel RY, White WM, et al. Epigenomic deconvolution of breast tumors reveals metabolic coupling between constituent cell types. Cell Rep. 2016;17:2075–86.
Chan RF, Turecki G, Shabalin AA, Guintivano J, Zhao M, Xie LY, et al. Cell Type-Specific Methylome-wide Association Studies Implicate Neurotrophin and Innate Immune Signaling in Major Depressive Disorder. Biol Psychiatry. 2020;87:431–42.
Shen-Orr SS, Tibshirani R, Khatri P, Bodian DL, Staedtler F, Perry NM, et al. Cell type-specific gene expression differences in complex tissues. Nat Methods. 2010;7:287–9.
Costello EJ, Angold A, Burns B, Stangl D, Tweed D, Erkanli A, et al. The Great Smoky Mountains Study of Youth: Goals, designs, methods, and the prevalence of DSM-III-R disorders. Arch Gen Psychiatry. 1996;53:1129–36.
Levey DF, Gelernter J, Polimanti R, Zhou H, Cheng Z, Aslan M, et al. Reproducible Genetic Risk Loci for Anxiety: Results From approximately 200,000 Participants in the Million Veteran Program. Am J Psychiatry. 2020;177:223–32.
Purves KL, Coleman JRI, Meier SM, Rayner C, Davis KAS, Cheesman R, et al. A major role for common genetic variation in anxiety disorders. Mol Psychiatry. 2020;25:3292–303.
Nagel M, Jansen PR, Stringer S, Watanabe K, de Leeuw CA, Bryois J, et al. Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat Genet. 2018;50:920–7.
Nievergelt CM, Maihofer AX, Klengel T, Atkinson EG, Chen CY, Choi KW, et al. International meta-analysis of PTSD genome-wide association studies identifies sex- and ancestry-specific genetic risk loci. Nat Commun. 2019;10:4558.
Kamburov A, Pentchev K, Galicka H, Wierling C, Lehrach H, Herwig R. ConsensusPathDB: toward a more complete picture of cell biology. Nucleic Acids Res. 2011;39:D712–7.
Thorp JG, Campos AI, Grotzinger AD, Gerring ZF, An J, Ong JS, et al. Symptom-level modelling unravels the shared genetic architecture of anxiety and depression. Nat Hum Behav. 2021;5:1432–42.
Gronska-Peski M, Schachner M, Hebert JM. L1cam curbs the differentiation of adult-born hippocampal neurons. Stem Cell Res. 2020;48:101999.
Huang J, Bonni A. A decade of the anaphase-promoting complex in the nervous system. Genes Dev. 2016;30:622–38.
Delgado-Esteban M, Garcia-Higuera I, Maestre C, Moreno S, Almeida A. APC/C-Cdh1 coordinates neurogenesis and cortical size during development. Nat Commun. 2013;4:2879.
Bobo-Jimenez V, Delgado-Esteban M, Angibaud J, Sanchez-Moran I, de la Fuente A, Yajeya J, et al. APC/C(Cdh1)-Rock2 pathway controls dendritic integrity and memory. Proc Natl Acad Sci USA. 2017;114:4513–8.
Almeida A. Regulation of APC/C-Cdh1 and its function in neuronal survival. Mol Neurobiol. 2012;46:547–54.
Pick JE, Malumbres M, Klann E. The E3 ligase APC/C-Cdh1 is required for associative fear memory and long-term potentiation in the amygdala of adult mice. Learn Mem. 2012;20:11–20.
Pick JE, Wang L, Mayfield JE, Klann E. Neuronal expression of the ubiquitin E3 ligase APC/C-Cdh1 during development is required for long-term potentiation, behavioral flexibility, and extinction. Neurobiol Learn Mem. 2013;100:25–31.
Gimenez-Llort L, Santana-Santana M, Bayascas JR. The Impact of the PI3K/Akt Signaling Pathway in Anxiety and Working Memory in Young and Middle-Aged PDK1 K465E Knock-In Mice. Front Behav Neurosci. 2020;14:61.
Qiao X, Gai H, Su R, Deji C, Cui J, Lai J, et al. PI3K-AKT-GSK3beta-CREB signaling pathway regulates anxiety-like behavior in rats following alcohol withdrawal. J Affect Disord. 2018;235:96–104.
Xiao X, Shang X, Zhai B, Zhang H, Zhang T. Nicotine alleviates chronic stress-induced anxiety and depressive-like behavior and hippocampal neuropathology via regulating autophagy signaling. Neurochem Int. 2018;114:58–70.
Zhang XH, Shen CL, Wang XY, Xiong WF, Shang X, Tang LY, et al. Increased Anxiety-like Behaviors in Adgra1(-/-) Male But Not Female Mice are Attributable to Elevated Neuron Dendrite Density, Upregulated PSD95 Expression, and Abnormal Activation of the PI3K/AKT/GSK-3beta and MEK/ERK Pathways. Neuroscience 2022;503:131–45.
Jin Q, Li J, Chen GY, Wu ZY, Liu XY, Liu Y, et al. Network and Experimental Pharmacology to Decode the Action of Wendan Decoction Against Generalized Anxiety Disorder. Drug Des Devel Ther. 2022;16:3297–314.
Ma Q, Zhou J, Yang Z, Xue Y, Xie X, Li T, et al. Mingmu Xiaoyao granules regulate the PI3K/Akt/mTOR signaling pathway to reduce anxiety and depression and reverse retinal abnormalities in rats. Front Pharm. 2022;13:1003614.
Niraula A, Witcher KG, Sheridan JF, Godbout JP. Interleukin-6 Induced by Social Stress Promotes a Unique Transcriptional Signature in the Monocytes That Facilitate Anxiety. Biol Psychiatry. 2019;85:679–89.
Cluny NL, Nyuyki KD, Almishri W, Griffin L, Lee BH, Hirota SA, et al. Recruitment of alpha4beta7 monocytes and neutrophils to the brain in experimental colitis is associated with elevated cytokines and anxiety-like behavior. J Neuroinflamm. 2022;19:73.
Guintivano J, Shabalin AA, Chan RF, Rubinow DR, Sullivan PF, Meltzer-Brody S, et al. Test-statistic inflation in methylome-wide association studies. Epigenetics 2020;15:1163–6.
Subelj L, Bajec M. Unfolding communities in large complex networks: combining defensive and offensive label propagation for core extraction. Phys Rev E Stat Nonlin Soft Matter Phys. 2011;83:036103.
Acknowledgements
This study was supported by the National Institutes of Health (R01MH099110 & 1R01MH104576 to E.J.C.G., 5R01MH113665 to J.M.H, R01MH109525 to K.A. and 1R01AA026057 to S.L.C.). The funding sources had no role in the study design, writing of the report or decision to submit the article for publication. The infrastructure for the NESDA study (www.nesda.nl) is funded through the Geestkracht program of the Netherlands Organisation for Health Research and Development (ZonMw, grant number 10-000-1002) and financial contributions by participating universities and mental health care organizations (VU University Medical Center, GGZ inGeest, Leiden University Medical Center, Leiden University, GGZ Rivierduinen, University Medical Center Groningen, University of Groningen, Lentis, GGZ Friesland, GGZ Drenthe, Rob Giel Onderzoekscentrum).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hettema, J.M., van den Oord, E.J.C.G., Zhao, M. et al. Methylome-wide association study of anxiety disorders. Mol Psychiatry 28, 3484–3492 (2023). https://doi.org/10.1038/s41380-023-02205-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02205-w