Abstract
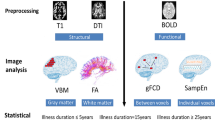
Although gray matter (GM) abnormalities are present from the early stages of psychosis, subtle/miniscule changes may not be detected by conventional volumetry. Texture analysis (TA), which permits quantification of the complex interrelationship between contrasts at the individual voxel level, may capture subtle GM changes with more sensitivity than does volume or cortical thickness (CTh). We performed three-dimensional TA in nine GM regions of interest (ROIs) using T1 magnetic resonance images from 101 patients with first-episode psychosis (FEP), 85 patients at clinical high risk (CHR) for psychosis, and 147 controls. Via principal component analysis, three features of gray-level cooccurrence matrix – informational measure of correlation 1 (IMC1), autocorrelation (AC), and inverse difference (ID) – were selected to analyze cortical texture in the ROIs that showed a significant change in volume or CTh in the study groups. Significant reductions in GM volume and CTh of various frontotemporal regions were found in the FEP compared with the controls. Increased frontal AC was found in the FEP group compared to the controls after adjusting for volume and CTh changes. While volume and CTh were preserved in the CHR group, a stagewise nonlinear increase in frontal IMC1 was found, which exceeded both the controls and FEP group. Increased frontal IMC1 was also associated with a lesser severity of attenuated positive symptoms in the CHR group, while neither volume nor CTh was. The results of the current study suggest that frontal IMC1 may reflect subtle, dynamic GM changes and the symptomatology of the CHR stage with greater sensitivity, even in the absence of gross GM abnormalities. Some structural mechanisms that may contribute to texture changes (e.g., macrostructural cortical lamina, neuropil/myelination, cortical reorganization) and their possible implications are explored and discussed. Texture may be a useful tool to investigate subtle and dynamic GM abnormalities, especially during the CHR period.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho B-C. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry. 2011;70:672–9.
Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry. 2012;2:e190.
Van Haren NE, Schnack HG, Cahn W, Van Den Heuvel MP, Lepage C, Collins L, et al. Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry. 2011;68:871–80.
Zipursky RB, Lambe EK, Kapur S, Mikulis DJ. Cerebral gray matter volume deficits in first episode psychosis. Arch Gen Psychiatry. 1998;55:540–6.
Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Structural gray matter differences between first-episode schizophrenics and normal controls using voxel-based morphometry. Neuroimage. 2002;17:880–9.
Hulshoff Pol HE, Kahn RS. What happens after the first episode? A review of progressive brain changes in chronically ill patients with schizophrenia. Schizophr Bull. 2008;34:354–66.
Fusar-Poli P, Borgwardt S, Crescini A, Deste G, Kempton MJ, Lawrie S, et al. Neuroanatomy of vulnerability to psychosis: a voxel-based meta-analysis. Neurosci Biobehav Rev. 2011;35:1175–85.
Mechelli A, Riecher-Rössler A, Meisenzahl EM, Tognin S, Wood SJ, Borgwardt SJ, et al. Neuroanatomical abnormalities that predate the onset of psychosis: a multicenter study. Arch Gen Psychiatry. 2011;68:489–95.
Iwashiro N, Suga M, Takano Y, Inoue H, Natsubori T, Satomura Y, et al. Localized gray matter volume reductions in the pars triangularis of the inferior frontal gyrus in individuals at clinical high-risk for psychosis and first episode for schizophrenia. Schizophr Res. 2012;137:124–31.
Ding Y, Ou Y, Pan P, Shan X, Chen J, Liu F, et al. Brain structural abnormalities as potential markers for detecting individuals with ultra-high risk for psychosis: a systematic review and meta-analysis. Schizophr Res. 2019;209:22–31.
Zikidi K, Gajwani R, Gross J, Gumley AI, Lawrie SM, Schwannauer M, et al. Grey-matter abnormalities in clinical high-risk participants for psychosis. Schizophr Res. 2020;226:120–8.
Cropley VL, Lin A, Nelson B, Reniers RL, Yung AR, Bartholomeusz CF, et al. Baseline grey matter volume of non-transitioned “ultra high risk” for psychosis individuals with and without attenuated psychotic symptoms at long-term follow-up. Schizophr Res. 2016;173:152–8.
Chung Y, Jacobson A, He G, van Erp TG, McEwen S, Addington J, et al. Prodromal symptom severity predicts accelerated gray matter reduction and third ventricle expansion among clinically high-risk youth developing psychotic disorders. Complex Psychiatry. 2015;1:13–22.
Walter A, Studerus E, Smieskova R, Kuster P, Aston J, Lang UE, et al. Hippocampal volume in subjects at high risk of psychosis: a longitudinal MRI study. Schizophr Res. 2012;142:217–22.
Lee S, Lee H, Kim KW. Magnetic resonance imaging texture predicts progression to dementia due to Alzheimer disease earlier than hippocampal volume. J Psychiatry Neurosci. 2020;45:7–14.
Depeursinge A, Foncubierta-Rodriguez A, Van De Ville D, Müller H. Three-dimensional solid texture analysis in biomedical imaging: review and opportunities. Med Image Anal. 2014;18:176–96.
Aerts HJ. The potential of radiomic-based phenotyping in precision medicine: a review. JAMA Oncol. 2016;2:1636–42.
Fan Y, Feng M, Wang R. Application of radiomics in central nervous system diseases: a systematic literature review. Clin Neurol Neurosurg. 2019;187:105565.
Zhang Y, Zhu H, Mitchell JR, Costello F, Metz LM. T2 MRI texture analysis is a sensitive measure of tissue injury and recovery resulting from acute inflammatory lesions in multiple sclerosis. Neuroimage. 2009;47:107–11.
Holli KK, Wäljas M, Harrison L, Liimatainen S, Luukkaala T, Ryymin P, et al. Mild traumatic brain injury: tissue texture analysis correlated to neuropsychological and DTI findings. Acad Radiol. 2010;17:1096–102.
Ishaque A, Mah D, Seres P, Luk C, Johnston W, Chenji S, et al. Corticospinal tract degeneration in ALS unmasked in T1‐weighted images using texture analysis. Hum Brain Mapp. 2019;40:1174–83.
Sørensen L, Igel C, Liv Hansen N, Osler M, Lauritzen M, Rostrup E, et al. Early detection of Alzheimer’s disease using M RI hippocampal texture. Hum Brain Mapp. 2016;37:1148–61.
Kassner A, Thornhill R. Texture analysis: a review of neurologic MR imaging applications. Am J Neuroradiol. 2010;31:809–16.
Ganeshan B, Miles KA, Young RC, Chatwin CR, Gurling HM, Critchley HD. Three-dimensional textural analysis of brain images reveals distributed grey-matter abnormalities in schizophrenia. Eur Radiol. 2010;20:941–8.
Radulescu E, Ganeshan B, Shergill SS, Medford N, Chatwin C, Young RC, et al. Grey-matter texture abnormalities and reduced hippocampal volume are distinguishing features of schizophrenia. Psychiatry Res Neuroimaging. 2014;223:179–86.
Latha M, Kavitha G. Segmentation and texture analysis of structural biomarkers using neighborhood-clustering-based level set in MRI of the schizophrenic brain. Magn Reson Mater Phys Biol Med. 2018;31:483–99.
Korda A, Ruef A, Neufang S, Davatzikos C, Borgwardt S, Meisenzahl E, et al. Identification of voxel-based texture abnormalities as new biomarkers for schizophrenia and major depressive patients using layer-wise relevance propagation on deep learning decisions. Psychiatry Res Neuroimaging. 2021;313:111303.
Korda AI, Andreou C, Rogg HV, Avram M, Ruef A, Davatzikos C, et al. Identification of texture MRI brain abnormalities on first-episode psychosis and clinical high-risk subjects using explainable artificial intelligence. Transl Psychiatry. 2022;12:481.
Park YW, Choi D, Lee J, Ahn SS, Lee S-K, Lee S-H, et al. Differentiating patients with schizophrenia from healthy controls by hippocampal subfields using radiomics. Schizophr Res. 2020;223:337–44.
Jalbrzikowski M, Hayes RA, Wood SJ, Nordholm D, Zhou JH, Fusar-Poli P, et al. Association of structural magnetic resonance imaging measures with psychosis onset in individuals at clinical high risk for developing psychosis: an ENIGMA working group mega-analysis: an ENIGMA working group mega-analysis. JAMA Psychiatry. 2021;78:753–66.
Lee S, Kim KW. Initiative ftAsDN. Associations between texture of T1-weighted magnetic resonance imaging and radiographic pathologies in Alzheimer’s disease. Eur J Neurol. 2021;28:735–44.
Lee TY, Hwang WJ, Kim NS, Park I, Lho SK, Moon S-Y, et al. Prediction of psychosis: model development and internal validation of a personalized risk calculator. Psychol Med. 2020;52:1–9.
Yi J-S, Ahn Y-M, Shin H-K, An S-K, Joo Y-H, Kim S-H, et al. Reliability and validity of the Korean version of the Positive and Negative Syndrome Scale. J Korean Neuropsychiatr Assoc. 2001;40:1090–105.
Jung MH, Jang JH, Kang D-H, Choi J-S, Shin NY, Kim HS, et al. The reliability and validity of the Korean version of the structured interview for prodromal syndrome. Psychiatry Investig. 2010;7:257.
Lobbestael J, Leurgans M, Arntz A. Inter‐rater reliability of the Structured Clinical Interview for DSM‐IV Axis I disorders (SCID I) and Axis II disorders (SCID II). Clin Psychol Psychother. 2011;18:75–9.
Schwarz CG, Gunter JL, Wiste HJ, Przybelski SA, Weigand SD, Ward CP, et al. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer’s disease severity. NeuroImage Clin. 2016;11:802–12.
Haralick RM, Shanmugam K, Dinstein IH. Textural features for image classification. IEEE Trans Syst Man Cybern. 1973;6:610–21.
Collewet G, Strzelecki M, Mariette F. Influence of MRI acquisition protocols and image intensity normalization methods on texture classification. Magn Reson Imaging. 2004;22:81–91.
Mahmoud-Ghoneim D, Alkaabi MK, de Certaines JD, Goettsche F-M. The impact of image dynamic range on texture classification of brain white matter. BMC Med Imaging. 2008;8:1–8.
Ortiz A, Palacio AA, Górriz JM, Ramírez J, Salas-González D. Segmentation of brain MRI using SOM-FCM-based method and 3D statistical descriptors. Comput Math Methods Med. 2013;2013.
Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–93.
Dukart J, Smieskova R, Harrisberger F, Lenz C, Schmidt A, Walter A, et al. Age-related brain structural alterations as an intermediate phenotype of psychosis. J Psychiatry Neurosci JPN. 2017;42:307.
Gupta CN, Calhoun VD, Rachakonda S, Chen J, Patel V, Liu J, et al. Patterns of gray matter abnormalities in schizophrenia based on an international mega-analysis. Schizophr Bull. 2015;41:1133–42.
Wolkin A, Rusinek H, Vaid G, Arena L, Lafargue T, Sanfilipo M, et al. Structural magnetic resonance image averaging in schizophrenia. Am J Psychiatry. 1998;155:1064–73.
Lawrie S, Abukmeil S, Chiswick A, Egan V, Santosh C, Best J. Qualitative cerebral morphology in schizophrenia: a magnetic resonance imaging study and systematic literature review. Schizophr Res. 1997;25:155–66.
Rosa P, Zanetti M, Duran F, Santos L, Menezes P, Scazufca M, et al. What determines continuing grey matter changes in first-episode schizophrenia and affective psychosis? Psychol Med. 2015;45:817–28.
van Haren NE, Cahn W, Pol HH, Kahn R. Schizophrenia as a progressive brain disease. Eur Psychiatry. 2008;23:245–54.
Does MD. Inferring brain tissue composition and microstructure via MR relaxometry. NeuroImage. 2018;182:136–48.
Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci. 2000;97:11050–5.
Sprooten E, O’Halloran R, Dinse J, Lee WH, Moser DA, Doucet GE, et al. Depth-dependent intracortical myelin organization in the living human brain determined by in vivo ultra-high field magnetic resonance imaging. NeuroImage. 2019;185:27–34.
Harrison PJ. The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain. 1999;122:593–624.
Wagstyl K, Ronan L, Whitaker K, Goodyer I, Roberts N, Crow T, et al. Multiple markers of cortical morphology reveal evidence of supragranular thinning in schizophrenia. Transl Psychiatry. 2016;6:e780.
Williams M, Chaudhry R, Perera S, Pearce R, Hirsch S, Ansorge O, et al. Changes in cortical thickness in the frontal lobes in schizophrenia are a result of thinning of pyramidal cell layers. Eur Arch Psychiatry Clin Neurosci. 2013;263:25–39.
Wiegand LC, Warfield SK, Levitt JJ, Hirayasu Y, Salisbury DF, Heckers S, et al. Prefrontal cortical thickness in first-episode psychosis: a magnetic resonance imaging study. Biol Psychiatry. 2004;55:131–40.
Bakhshi K, Chance S. The neuropathology of schizophrenia: a selective review of past studies and emerging themes in brain structure and cytoarchitecture. Neurosci. 2015;303:82–102.
Harrison PJ. Postmortem studies in schizophrenia. Dialog Clin Neurosci. 2022;2:349–57.
Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–75.
Kolomeets NS, Uranova NA. Reduced oligodendrocyte density in layer 5 of the prefrontal cortex in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2019;269:379–86.
Foong J, Symms M, Barker G, Maier M, Woermann F, Miller D, et al. Neuropathological abnormalities in schizophrenia: evidence from magnetization transfer imaging. Brain. 2001;124:882–92.
Price G, Cercignani M, Chu EM, Barnes TR, Barker GJ, Joyce EM, et al. Brain pathology in first-episode psychosis: magnetization transfer imaging provides additional information to MRI measurements of volume loss. Neuroimage. 2010;49:185–92.
Maani R, Yang YH, Kalra S. Voxel-based texture analysis of the brain. PLoS One. 2015;10:e0117759.
Maani R, Yang Y-H, Emery D, Kalra S. Cerebral degeneration in amyotrophic lateral sclerosis revealed by 3-dimensional texture analysis. Front. Neurosci. 2016;10:120.
Tak K, Lee S, Choi E, Suh SW, Oh DJ, Moon W, et al. Magnetic resonance imaging texture of medial pulvinar in dementia with lewy bodies. Dement Geriatr Cogn Disord. 2020;49:8–15.
Sui YV, Bertisch H, Lee H-H, Storey P, Babb JS, Goff DC, et al. Quantitative macromolecular proton fraction mapping reveals altered cortical myelin profile in schizophrenia spectrum disorders. Cereb Cortex Commun. 2021;2:tgab015.
Rowley CD, Sehmbi M, Bazin PL, Tardif CL, Minuzzi L, Frey BN, et al. Age‐related mapping of intracortical myelin from late adolescence to middle adulthood using T1‐weighted MRI. Hum Brain Mapp. 2017;38:3691–703.
Edwards LJ, Kirilina E, Mohammadi S, Weiskopf N. Microstructural imaging of human neocortex in vivo. Neuroimage. 2018;182:184–206.
Palaniyappan L. Progressive cortical reorganisation: a framework for investigating structural changes in schizophrenia. Neurosci Biobehav Rev. 2017;79:1–13.
Abel S, Weiller C, Huber W, Willmes K, Specht K. Therapy-induced brain reorganization patterns in aphasia. Brain. 2015;138:1097–112.
Kerr AL, Cheng S-Y, Jones TA. Experience-dependent neural plasticity in the adult damaged brain. J Commun Disord. 2011;44:538–48.
Westlye LT, Walhovd KB, Dale AM, Bjørnerud A, Due-Tønnessen P, Engvig A, et al. Differentiating maturational and aging-related changes of the cerebral cortex by use of thickness and signal intensity. Neuroimage. 2010;52:172–85.
Palaniyappan L, Das T, Dempster K. The neurobiology of transition to psychosis: clearing the cache. J Psychiatry Neurosci. 2017;42:294–9.
Aerts HJ, Velazquez ER, Leijenaar RT, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5:1–9.
Van Griethuysen JJ, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77:e104–e107.
Moyer CE, Shelton MA, Sweet RA. Dendritic spine alterations in schizophrenia. Neurosci Lett. 2015;601:46–53.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) and the KBRI basic research program through Korea Brain Research Institute, funded by the Ministry of Science & ICT (grant nos. 2019R1C1C1002457, 2020M3E5D9079910, and 21-BR-03-01).
Author information
Authors and Affiliations
Contributions
JSK, KWK, and SYM conceived the project. SYM designed the study methodology and wrote the first manuscript with the help of all other authors. HP and WL performed MRI and texture analysis with the help of SL, KWK, and SYM. MK and JSK provided the resources and supervised the project. Review and editing of the first manuscript were performed by MK, JSK, and SYM.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moon, S.Y., Park, H., Lee, W. et al. Magnetic resonance texture analysis reveals stagewise nonlinear alterations of the frontal gray matter in patients with early psychosis. Mol Psychiatry 28, 5309–5318 (2023). https://doi.org/10.1038/s41380-023-02163-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02163-3