Abstract
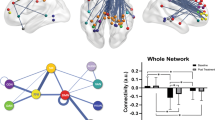
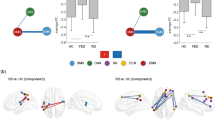
The effect of antipsychotic medication on resting state functional connectivity in major depressive disorder (MDD) is currently unknown. To address this gap, we examined patients with MDD with psychotic features (MDDPsy) participating in the Study of the Pharmacotherapy of Psychotic Depression II. All participants were treated with sertraline plus olanzapine and were subsequently randomized to continue sertraline plus olanzapine or be switched to sertraline plus placebo. Participants completed an MRI at randomization and at study endpoint (study completion at Week 36, relapse, or early termination). The primary outcome was change in functional connectivity measured within and between specified networks and the rest of the brain. The secondary outcome was change in network topology measured by graph metrics. Eighty-eight participants completed a baseline scan; 73 completed a follow-up scan, of which 58 were usable for analyses. There was a significant treatment X time interaction for functional connectivity between the secondary visual network and rest of the brain (t = −3.684; p = 0.0004; pFDR = 0.0111). There was no significant treatment X time interaction for graph metrics. Overall, functional connectivity between the secondary visual network and the rest of the brain did not change in participants who stayed on olanzapine but decreased in those switched to placebo. There were no differences in changes in network topology measures when patients stayed on olanzapine or switched to placebo. This suggests that olanzapine may stabilize functional connectivity, particularly between the secondary visual network and the rest of the brain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Code availability
Code used for these analyses can be found here: https://github.com/loliver4/STOPPD_Long_FC
References
Marder SR, Cannon TD. Schizophrenia. N Engl J Med. 2019;381:1753–61.
Farahani A, Correll CU. Are antipsychotics or antidepressants needed for psychotic depression? A systematic review and meta-analysis of trials comparing antidepressant or antipsychotic monotherapy with combination treatment. J Clin Psychiatry. 2012;73:486–96.
Meyers BS, Flint AJ, Rothschild AJ, Mulsant BH, Whyte EM, Peasley-Miklus C, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry. 2009;66:838–47.
Flint AJ, Meyers BS, Rothschild AJ, Whyte EM, Alexopoulos GS, Rudorfer MV, et al. Effect of Continuing Olanzapine vs Placebo on Relapse Among Patients With Psychotic Depression in Remission: The STOP-PD II Randomized Clinical Trial. JAMA. 2019;322:622–31.
Ho B-C, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68:128–37.
Lesh TA, Tanase C, Geib BR, Niendam TA, Yoon JH, Minzenberg MJ, et al. A multimodal analysis of antipsychotic effects on brain structure and function in first-episode schizophrenia. JAMA Psychiatry. 2015;72:226–34.
Szeszko PR, Robinson DG, Ikuta T, Peters BD, Gallego JA, Kane J, et al. White matter changes associated with antipsychotic treatment in first-episode psychosis. Neuropsychopharmacology. 2014;39:1324–31.
Voineskos AN, Mulsant BH, Dickie EW, Neufeld NH, Rothschild AJ, Whyte EM, et al. Effects of Antipsychotic Medication on Brain Structure in Patients With Major Depressive Disorder and Psychotic Features: Neuroimaging Findings in the Context of a Randomized Placebo-Controlled Clinical Trial. JAMA Psychiatry. 2020;77:674–83.
Konopaske GT, Dorph-Petersen K-A, Pierri JN, Wu Q, Sampson AR, Lewis DA. Effect of chronic exposure to antipsychotic medication on cell numbers in the parietal cortex of macaque monkeys. Neuropsychopharmacology. 2007;32:1216–23.
Konopaske GT, Dorph-Petersen K-A, Sweet RA, Pierri JN, Zhang W, Sampson AR, et al. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol Psychiatry. 2008;63:759–65.
Vernon AC, Natesan S, Modo M, Kapur S. Effect of chronic antipsychotic treatment on brain structure: a serial magnetic resonance imaging study with ex vivo and postmortem confirmation. Biol Psychiatry. 2011;69:936–44.
Vernon AC, Crum WR, Lerch JP, Chege W, Natesan S, Modo M, et al. Reduced Cortical Volume and Elevated Astrocyte Density in Rats Chronically Treated With Antipsychotic Drugs—Linking Magnetic Resonance Imaging Findings to Cellular Pathology. Biol Psychiatry. 2014;75:982–90.
Neufeld NH, Mulsant BH, Dickie EW, Meyers BS, Alexopoulos GS, Rothschild AJ, et al. Resting state functional connectivity in patients with remitted psychotic depression: A multi-centre STOP-PD study. EBioMedicine. 2018;36:446–53.
Sudheimer K, Keller J, Gomez R, Tennakoon L, Reiss A, Garrett A, et al. Decreased hypothalamic functional connectivity with subgenual cortex in psychotic major depression. Neuropsychopharmacology. 2015;40:849–60.
Oudega ML, van der Werf YD, Dols A, Wattjes MP, Barkhof F, Bouckaert F, et al. Exploring resting state connectivity in patients with psychotic depression. PLoS One. 2019;14:e0209908.
Esteban O, Markiewicz CJ, Blair RW, Moodie CA, Isik AI, Erramuzpe A, et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat Methods. 2019;16:111–6.
Dickie EW, Anticevic A, Smith DE, Coalson TS, Manogaran M, Calarco N, et al. Ciftify: A framework for surface-based analysis of legacy MR acquisitions. Neuroimage. 2019;197:818–26.
Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, et al. A multi-modal parcellation of human cerebral cortex. Nature. 2016;536:171–8.
Tian Y, Margulies DS, Breakspear M, Zalesky A. Topographic organization of the human subcortex unveiled with functional connectivity gradients. Nat Neurosci. 2020;23:1421–32.
Ji JL, Spronk M, Kulkarni K, Repovš G, Anticevic A, Cole MW. Mapping the human brain’s cortical-subcortical functional network organization. Neuroimage. 2019;185:35–57.
R Core Team. R: A Language and Environment for Statistical Computing. 2021.
Bertolero MA, Yeo BTT, D’Esposito M. The modular and integrative functional architecture of the human brain. Proc Natl Acad Sci USA. 2015;112:E6798–E6807.
Hugdahl K, Rund BR, Lund A, Asbjørnsen A, Egeland J, Ersland L, et al. Brain activation measured with fMRI during a mental arithmetic task in schizophrenia and major depression. Am J Psychiatry. 2004;161:286–93.
Del Fabro L, Delvecchio G, D’Agostino A, Brambilla P. Effects of olanzapine during cognitive and emotional processing in schizophrenia: A review of functional magnetic resonance imaging findings. Hum Psychopharmacol. 2019;34:e2693.
O’Donoghue B, Francey SM, Nelson B, Ratheesh A, Allott K, Graham J, et al. Staged treatment and acceptability guidelines in early psychosis study (STAGES): A randomized placebo controlled trial of intensive psychosocial treatment plus or minus antipsychotic medication for first-episode psychosis with low-risk of self-harm or aggression. Study protocol and baseline characteristics of participants. Early Interv Psychiatry. 2019;13:953–60.
Chopra S, Fornito A, Francey SM, O’Donoghue B, Cropley V, Nelson B, et al. Differentiating the effect of antipsychotic medication and illness on brain volume reductions in first-episode psychosis: A Longitudinal, Randomised, Triple-blind, Placebo-controlled MRI Study. Neuropsychopharmacology. 2021;46:1494–501.
Chopra S, Francey SM, O’Donoghue B, Sabaroedin K, Arnatkeviciute A, Cropley V, et al. Functional Connectivity in Antipsychotic-Treated and Antipsychotic-Naive Patients With First-Episode Psychosis and Low Risk of Self-harm or Aggression: A Secondary Analysis of a Randomized Clinical Trial. JAMA Psychiatry. 2021;78:994–1004.
Veer IM, Beckmann CF, van Tol M-J, Ferrarini L, Milles J, Veltman DJ, et al. Whole brain resting-state analysis reveals decreased functional connectivity in major depression. Front Syst Neurosci. 2010;4:41.
Yan C-G, Chen X, Li L, Castellanos FX, Bai T-J, Bo Q-J, et al. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc Natl Acad Sci USA. 2019;116:9078–83.
Javaheripour N, Li M, Chand T, Krug A, Kircher T, Dannlowski U, et al. Altered resting-state functional connectome in major depressive disorder: a mega-analysis from the PsyMRI consortium. Transl Psychiatry. 2021;11:511.
Martínez A, Hillyard SA, Dias EC, Hagler DJ Jr, Butler PD, Guilfoyle DN, et al. Magnocellular pathway impairment in schizophrenia: evidence from functional magnetic resonance imaging. J Neurosci. 2008;28:7492–7500.
Dias EC, Butler PD, Hoptman MJ, Javitt DC. Early Sensory Contributions to Contextual Encoding Deficits in Schizophrenia. Archives of General Psychiatry. 2011;68:654.
McCleery A, Wynn JK, Lee J, Reavis EA, Ventura J, Subotnik KL, et al. Early Visual Processing Is Associated With Social Cognitive Performance in Recent-Onset Schizophrenia. Front Psychiatry. 2020;11:823.
Schutte MJL, Bohlken MM, Collin G, Abramovic L, Boks MPM, Cahn W, et al. Functional connectome differences in individuals with hallucinations across the psychosis continuum. Sci Rep. 2021;11:1108.
Wang L-X, Guo F, Zhu Y-Q, Wang H-N, Liu W-M, Li C, et al. Effect of second-generation antipsychotics on brain network topology in first-episode schizophrenia: A longitudinal rs-fMRI study. Schizophr Res. 2019;208:160–6.
Nelson EA, Kraguljac NV, Maximo JO, Armstrong W, Lahti AC. Hippocampal Hyperconnectivity to the Visual Cortex Predicts Treatment Response. Schizophr Bull. 2023. https://doi.org/10.1093/schbul/sbac213.
Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychol Bull. 2013;139:81–132.
Flint AJ, Bingham KS, Neufeld NH, Alexopoulos GS, Mulsant BH, Rothschild AJ, et al. Association between psychomotor disturbance and treatment outcome in psychotic depression: a STOP-PD II report. Psychol Med. 2022;52:3957–63.
Flint AJ, Bingham KS, Alexopoulos GS, Marino P, Mulsant BH, Neufeld NH, et al. Predictors of relapse of psychotic depression: Findings from the STOP-PD II randomized clinical trial. J Psychiatr Res. 2023;157:285–90.
Bingham KS, Neufeld NH, Alexopoulos GS, Marino P, Mulsant BH, Rothschild AJ, et al. Factor analysis of the CORE measure of psychomotor disturbance in psychotic depression: Findings from the STOP-PD II study. Psychiatry Res. 2022;314:114648.
Kalkman HO. The role of the phosphatidylinositide 3-kinase–protein kinase B pathway in schizophrenia. Pharmacol Ther. 2006;110:117–34.
Albert KA, Hemmings HC Jr, Adamo AIB, Potkin SG, Akbarian S, Sandman CA, et al. Evidence for decreased DARPP-32 in the prefrontal cortex of patients with schizophrenia. Arch Gen Psychiatry. 2002;59:705–12.
Keshavan MS, Stanley JA, Montrose DM, Minshew NJ, Pettegrew JW. Prefrontal membrane phospholipid metabolism of child and adolescent offspring at risk for schizophrenia or schizoaffective disorder: an in vivo 31P MRS study. Mol Psychiatry. 2003;8:316–23. 251
Leppik L, Parksepp M, Janno S, Koido K, Haring L, Vasar E, et al. Profiling of lipidomics before and after antipsychotic treatment in first-episode psychosis. Eur Arch Psychiatry Clin Neurosci. 2020;270:59–70.
Song X, Chen X, Yuksel C, Yuan J, Pizzagalli DA, Forester B, et al. Bioenergetics and abnormal functional connectivity in psychotic disorders. Mol Psychiatry. 2021;26:2483–92.
MacDonald K, Krishnan A, Cervenka E, Hu G, Guadagno E, Trakadis Y. Biomarkers for major depressive and bipolar disorders using metabolomics: A systematic review. Am J Med Genet B Neuropsychiatr Genet. 2019;180:122–37.
Moriguchi S, Takamiya A, Noda Y, Horita N, Wada M, Tsugawa S, et al. Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry. 2019;24:952–64.
Volz H-P, Riehemann S, Maurer I, Smesny S, Sommer M, Rzanny R, et al. Reduced phosphodiesters and high-energy phosphates in the frontal lobe of schizophrenic patients: a 31P chemical shift spectroscopic-imaging study. Biological Psychiatry. 2000;47:954–61.
Wood SJ, Berger GE, Wellard RM, Proffitt T, McConchie M, Velakoulis D, et al. A 1H-MRS investigation of the medial temporal lobe in antipsychotic-naïve and early-treated first episode psychosis. Schizophr Res. 2008;102:163–70.
Scaini G, Rochi N, Morais MOS, Maggi DD, De-Nês BT, Quevedo J, et al. In vitro effect of antipsychotics on brain energy metabolism parameters in the brain of rats. Acta Neuropsychiatr. 2013;25:18–26.
Gordon EM, Laumann TO, Gilmore AW, Newbold DJ, Greene DJ, Berg JJ, et al. Precision Functional Mapping of Individual Human Brains. Neuron. 2017;95:791–807.e7.
Gratton C, Kraus BT, Greene DJ, Gordon EM, Laumann TO, Nelson SM, et al. Defining Individual-Specific Functional Neuroanatomy for Precision Psychiatry. Biol Psychiatry. 2020;88:28–39.
Oquendo MA, Baca-Garcia E, Kartachov A, Khait V, Campbell CE, Richards M, et al. A computer algorithm for calculating the adequacy of antidepressant treatment in unipolar and bipolar depression. J Clin Psychiatry. 2003;64:825–33.
Acknowledgements
This study was funded by the National Institute of Mental Health (NIMH) R01MH099167 grant. The STOP-PD II clinical trial from which participants were recruited was funded by US Public Health Service grants MH 62446, MH 62518, MH 62565, and MH 62624 from the NIMH. In that trial, Eli Lilly provided olanzapine and matching placebo pills and Pfizer provided sertraline; neither company provided funding for the study. ClinicalTrials.gov Identifier: NCT01427608. We thank the members of the STOP-PD II Study Group for their contributions. Members of the STOP-PD II Study Group were: Peter Giacobbe, M.D. and Brenda Swampillai B.Sc. at the University Health Network, Toronto and James Kennedy, M.D. and Bruce Pollock M.D., Ph.D. at the Centre for Addiction and Mental Health, Toronto; Kristina Deligiannidis, M.D., Chelsea Kosma, M.A., Wendy Marsh, M.D. at the University of Massachusetts Medical School and UMass Memorial Health Care, Worcester, MA; Ariel Gildengers M.D., Joelle Kincman Ph.D., Meryl Butters Ph.D., and Michelle Zmuda M.A. at the University of Pittsburgh School of Medicine, Pittsburgh, PA; and Judith English, M.A., James Kocsis, M.D., Barbara Ladenheim, Ph.D., Vassilios Latoussakis, M.D., and Nikhil Palekar, M.D. at Weill Cornell Medicine of Cornell University and New York Presbyterian Hospital, NY. We additionally thank Cristina Pollari, MPH, for her help with data management, Matthew Rudorfer, MD, who represented the NIMH on the STOP-PD II trial, and Judy Kwan, BSc, for her help with participant recruitment and assessment. We also thank all study participants.
Author information
Authors and Affiliations
Contributions
NHN, LDO, BHM, GSA, BSM, AJR, EMW, AJF, and ANV conceived and designed the study. All authors were involved in the acquisition, analysis, or interpretation of data. NHN, LDO, BHM, GSA, BSM, AJR, EMW, AJF, and ANV drafted the manuscript. All authors were involved in the critical revision of the manuscript for important intellectual content. NHN, LDO, and ANV contributed to statistical analyses. BHM, GSA, BSM, AJR, EMW, AJF, and ANV obtained funding. NHN, LDO, MJH, HT, PM, KSB, AJF, and ANV provided administrative, technical, or material support. ANV provided supervision.
Corresponding author
Ethics declarations
Competing interests
NHN receives grant support from the Brain and Behavior Research Foundation (BBRF), Canadian Institutes of Health Research (CIHR), Physicians’ Services Incorporated Foundation, Labatt Family Network for Research on the Biology of Depression, and the University of Toronto; this work was supported in part by an Academic Scholars Award from the Department of Psychiatry, University of Toronto. Dr Oliver receives grant support from the BBRF. BHM holds and receives support from the Labatt Family Chair in Biology of Depression in Late-Life Adults at the University of Toronto. He currently receives or has received research support during the past three years from Brain Canada, CIHR, CAMH Foundation, Patient-Centered Outcomes Research Institute (PCORI), US National Institutes of Health (NIH), Capital Solution Design LLC (software used in a study funded by CAMH Foundation), and HAPPYneuron (software used in a study funded by Brain Canada). Within the past three years, he has also been an unpaid consultant to Myriad Neuroscience. He directly owns stocks of General Electric (less than $5000). GSA has received National Institute of Mental Health (NIMH) grants and has served in the speakers bureau of Takeda, Lundbeck, Otsuka, Alergan, Astra/Zeneca, and Sunovion. MJH has received an American Foundation for Suicide Prevention grant and NIMH subcontract funding during the conduct of this study. HT was supported by a Postdoctoral Fellowship from CIHR. He has received manuscript or speaker fees from Dainippon Sumitomo Pharma, Janssen Pharmaceutical, Otsuka Pharmaceutical, Takeda, Wiley Japan, and Yoshitomi Yakuhin. PM received research support from the NIMH at the time this work was done. BSM received research support from the NIMH at the time this work was done. AJR has received grant or research support from Janssen, Otsuka, and the Irving S. and Betty Brudnick Endowed Chair in Psychiatry; is a consultant to Daiichi Sankyo, Inc., Sage Therapeutics, Xenon Pharmaceuticals, and Neumora Therapeutics; and has received royalties for the Rothschild Scale for Antidepressant Tachyphylaxis (RSAT)®, Clinical Manual for the Diagnosis and Treatment of Psychotic Depression, American Psychiatric Press, 2009; The Evidence-Based Guide to Antipsychotic Medications, American Psychiatric Press, 2010; The Evidence-Based Guide to Antidepressant Medications, American Psychiatric Press, 2012, and from UpToDate®. EMW receives grant support from the NIMH and Health Resources and Services Administration. KSB receives grant support from the University of Toronto. AJF has received grant support from the US NIH, PCORI, CIHR, Brain Canada, Ontario Brain Institute, Alzheimer’s Association, AGE-WELL, Canadian Foundation for Healthcare Improvement, and University of Toronto. ANV receives funding from the NIMH, CIHR, Canada Foundation for Innovation, CAMH Foundation, and the University of Toronto.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Neufeld, N.H., Oliver, L.D., Mulsant, B.H. et al. Effects of antipsychotic medication on functional connectivity in major depressive disorder with psychotic features. Mol Psychiatry 28, 3305–3313 (2023). https://doi.org/10.1038/s41380-023-02118-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02118-8
This article is cited by
-
Brain metabolite levels in remitted psychotic depression with consideration of effects of antipsychotic medication
Brain Imaging and Behavior (2023)