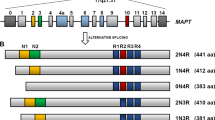
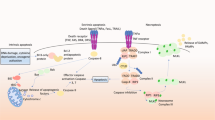
Abstract
Neuronal death is one of the most common pathological hallmarks of diverse neurological diseases, which manifest varying degrees of cognitive or motor dysfunction. Neuronal death can be classified into multiple forms with complicated and unique regulatory signaling pathways. Tau is a key microtubule-associated protein that is predominantly expressed in neurons to stabilize microtubules under physiological conditions. In contrast, pathological tau always detaches from microtubules and is implicated in a series of neurological disorders that are characterized by irreversible neuronal death, such as necrosis, apoptosis, necroptosis, pyroptosis, ferroptosis, autophagy-dependent neuronal death and phagocytosis by microglia. However, recent studies have also revealed that pathological tau can facilitate neuron escape from acute apoptosis, delay necroptosis through its action on granulovacuolar degeneration bodies (GVBs), and facilitate iron export from neurons to block ferroptosis. In this review, we briefly describe the current understanding of how pathological tau exerts dual effects on neuronal death by acting as a double-edged sword in different neurological diseases. We propose that elucidating the mechanism by which pathological tau affects neuronal death is critical for exploring novel and precise therapeutic strategies for neurological disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bredesen DE, Rao RV, Mehlen P. Cell death in the nervous system. Nature. 2006;443:796–802.
Fricker M, Tolkovsky AM, Borutaite V, Coleman M, Brown GC. Neuronal cell death. Physiol Rev. 2018;98:813–80.
West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344:769–72.
Schulz JB, Falkenburger BH. Neuronal pathology in Parkinson’s disease. Cell Tissue Res. 2004;318:135–47.
Guo Z, Rudow G, Pletnikova O, Codispoti KE, Orr BA, Crain BJ, et al. Striatal neuronal loss correlates with clinical motor impairment in Huntington’s disease. Mov Disord. 2012;27:1379–86.
Brettschneider J, Arai K, Del Tredici K, Toledo JB, Robinson JL, Lee EB, et al. TDP-43 pathology and neuronal loss in amyotrophic lateral sclerosis spinal cord. Acta Neuropathol. 2014;128:423–37.
Fung CW, Guo J, Fu H, Figueroa HY, Konofagou EE, Duff KE. Atrophy associated with tau pathology precedes overt cell death in a mouse model of progressive tauopathy. Sci Adv. 2020;6:eabc8098.
Gorman AM. Neuronal cell death in neurodegenerative diseases: recurring themes around protein handling. J Cell Mol Med. 2008;12:2263–80.
Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat Rev Neurosci. 2003;4:49–60.
Blokhuis AM, Groen EJ, Koppers M, van den Berg LH, Pasterkamp RJ. Protein aggregation in amyotrophic lateral sclerosis. Acta Neuropathol. 2013;125:777–94.
Di J, Cohen LS, Corbo CP, Phillips GR, El Idrissi A, Alonso AD. Abnormal tau induces cognitive impairment through two different mechanisms: synaptic dysfunction and neuronal loss. Sci Rep. 2016;6:20833.
van der Kant R, Goldstein LSB, Ossenkoppele R. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat Rev Neurosci. 2020;21:21–35.
Li X, Yu H, Zhang B, Li L, Chen W, Yu Q, et al. Molecularly defined and functionally distinct cholinergic subnetworks. Neuron. 2022;110:3774–88.e3777.
Ramsden M, Kotilinek L, Forster C, Paulson J, McGowan E, SantaCruz K, et al. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J Neurosci. 2005;25:10637–47.
Gómez-Isla T, Hollister R, West H, Mui S, Growdon JH, Petersen RC, et al. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann Neurol. 1997;41:17–24.
Wang Y, Mandelkow E. Tau in physiology and pathology. Nat Rev Neurosci. 2016;17:5–21.
Goedert M, Wischik CM, Crowther RA, Walker JE, Klug A. Cloning and sequencing of the cDNA encoding a core protein of the paired helical filament of Alzheimer disease: identification as the microtubule-associated protein tau. Proc Natl Acad Sci USA. 1988;85:4051–5.
Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5’-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–5.
Guo T, Noble W, Hanger DP. Roles of tau protein in health and disease. Acta Neuropathol. 2017;133:665–704.
Lassmann H, Bancher C, Breitschopf H, Wegiel J, Bobinski M, Jellinger K, et al. Cell death in Alzheimer’s disease evaluated by DNA fragmentation in situ. Acta Neuropathol. 1995;89:35–41.
Beard J. IV. On the early development of Lepidosteus osseus.—Preliminary notice. Proc R Soc Lond. 1890;46:108–18.
Collin R. Histolyse de certains neuroblastes au cours du développement du tube nerveux chez le poulet. CR Soc Biol. 1906;60:1080–1.
Lockshin RA, Williams CM. Programmed cell death—II. Endocrine potentiation of the breakdown of the intersegmental muscles of silkmoths. J Insect Physiol. 1964;10:643–9.
Kole AJ, Annis RP, Deshmukh M. Mature neurons: equipped for survival. Cell Death Dis. 2013;4:e689.
Esteras N, Kundel F, Amodeo GF, Pavlov EV, Klenerman D, Abramov AY. Insoluble tau aggregates induce neuronal death through modification of membrane ion conductance, activation of voltage-gated calcium channels and NADPH oxidase. FEBS J. 2021;288:127–41.
Kimura T, Fukuda T, Sahara N, Yamashita S, Murayama M, Mizoroki T, et al. Aggregation of detergent-insoluble tau is involved in neuronal loss but not in synaptic loss. J Biol Chem. 2010;285:38692–9.
West of Scotland Coronary Prevention Study: identification of high-risk groups and comparison with other cardiovascular intervention trials. Lancet. 1996;348:1339–42.
Brown GC. Neuronal Loss after Stroke Due to Microglial Phagocytosis of Stressed Neurons. Int J Mol Sci. 2021;22:13442.
Yamaguchi Y, Miura M. Programmed cell death in neurodevelopment. Dev cell. 2015;32:478–90.
Arendt T, Brückner MK, Morawski M, Jäger C, Gertz HJ. Early neurone loss in Alzheimer’s disease: cortical or subcortical? Acta Neuropathol Commun. 2015;3:10.
Bussière T, Giannakopoulos P, Bouras C, Perl DP, Morrison JH, Hof PR. Progressive degeneration of nonphosphorylated neurofilament protein-enriched pyramidal neurons predicts cognitive impairment in Alzheimer’s disease: stereologic analysis of prefrontal cortex area 9. J Comp Neurol. 2003;463:281–302.
Martínez-Pinilla E, Ordóñez C, Del Valle E, Navarro A, Tolivia J. Regional and gender study of neuronal density in brain during aging and in Alzheimer’s disease. Front aging Neurosci. 2016;8:213.
Seward ME, Swanson E, Norambuena A, Reimann A, Cochran JN, Li R, et al. Amyloid-β signals through tau to drive ectopic neuronal cell cycle re-entry in Alzheimer’s disease. J Cell Sci. 2013;126:1278–86. Pt 5.
Drubin DG, Kirschner MW. Tau protein function in living cells. J Cell Biol. 1986;103:2739–46. 6 Pt 2.
Dabir DV, Robinson MB, Swanson E, Zhang B, Trojanowski JQ, Lee VM, et al. Impaired glutamate transport in a mouse model of tau pathology in astrocytes. J Neurosci. 2006;26:644–54.
Seiberlich V, Bauer NG, Schwarz L, Ffrench-Constant C, Goldbaum O, Richter-Landsberg C. Downregulation of the microtubule associated protein tau impairs process outgrowth and myelin basic protein mRNA transport in oligodendrocytes. Glia. 2015;63:1621–35.
Pooler AM, Phillips EC, Lau DH, Noble W, Hanger DP. Physiological release of endogenous tau is stimulated by neuronal activity. EMBO Rep. 2013;14:389–94.
Yamada K, Holth JK, Liao F, Stewart FR, Mahan TE, Jiang H, et al. Neuronal activity regulates extracellular tau in vivo. J Exp Med. 2014;211:387–93.
Morris M, Maeda S, Vossel K, Mucke L. The many faces of tau. Neuron. 2011;70:410–26.
Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3:519–26.
Goedert M, Spillantini MG, Potier MC, Ulrich J, Crowther RA. Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J. 1989;8:393–9.
Liu C, Götz J. Profiling murine tau with 0N, 1N and 2N isoform-specific antibodies in brain and peripheral organs reveals distinct subcellular localization, with the 1N isoform being enriched in the nucleus. PloS One. 2013;8:e84849.
Alquezar C, Arya S, Kao AW. Tau post-translational modifications: dynamic transformers of Tau function, degradation, and aggregation. Front Neurol. 2020;11:595532.
Wesseling H, Mair W, Kumar M, Schlaffner CN, Tang S, Beerepoot P, et al. Tau PTM profiles identify patient heterogeneity and stages of Alzheimer’s disease. Cell. 2020;183:1699–713.e1613.
Shin S, Kim D, Song JY, Jeong H, Hyeon SJ, Kowall NW, et al. Visualization of soluble tau oligomers in TauP301L-BiFC transgenic mice demonstrates the progression of tauopathy. Progr Neurobiol. 2020;187:101782.
Noble W, Hanger DP, Miller CC, Lovestone S. The importance of tau phosphorylation for neurodegenerative diseases. Front Neurol. 2013;4:83.
Alonso AC, Zaidi T, Grundke-Iqbal I, Iqbal K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:5562–6.
Hoover BR, Reed MN, Su J, Penrod RD, Kotilinek LA, Grant MK, et al. Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68:1067–81.
Decker JM, Krüger L, Sydow A, Zhao S, Frotscher M, Mandelkow E, et al. Pro-aggregant Tau impairs mossy fiber plasticity due to structural changes and Ca(++) dysregulation. Acta Neuropathol Commun. 2015;3:23.
Pooler AM, Noble W, Hanger DP. A role for tau at the synapse in Alzheimer’s disease pathogenesis. Neuropharmacology. 2014;76:1–8. Pt A.
Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell. 2010;142:387–97.
Wu M, Zhang M, Yin X, Chen K, Hu Z, Zhou Q, et al. The role of pathological tau in synaptic dysfunction in Alzheimer’s diseases. Transl Neurodegener. 2021;10:45.
Pampuscenko K, Morkuniene R, Krasauskas L, Smirnovas V, Tomita T, Borutaite V. Distinct neurotoxic effects of extracellular Tau species in primary neuronal-glial cultures. Mol Neurobiol. 2021;58:658–67.
Andorfer C, Acker CM, Kress Y, Hof PR, Duff K, Davies P. Cell-cycle reentry and cell death in transgenic mice expressing nonmutant human tau isoforms. J Neurosci. 2005;25:5446–54.
Amadoro G, Ciotti MT, Costanzi M, Cestari V, Calissano P, Canu N. NMDA receptor mediates tau-induced neurotoxicity by calpain and ERK/MAPK activation. Proc Natl Acad Sci USA. 2006;103:2892–7.
Bano D, Ankarcrona M. Beyond the critical point: an overview of excitotoxicity, calcium overload and the downstream consequences. Neurosci Lett. 2018;663:79–85.
Ittner A, Chua SW, Bertz J, Volkerling A, van der Hoven J, Gladbach A, et al. Site-specific phosphorylation of tau inhibits amyloid-β toxicity in Alzheimer’s mice. Science. 2016;354:904–8.
Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57.
Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–9.
Dickson DW. Apoptotic mechanisms in Alzheimer neurofibrillary degeneration: cause or effect? J Clin Investig. 2004;114:23–27.
Green DR. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102:1–4.
Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541.
Su JH, Zhao M, Anderson AJ, Srinivasan A, Cotman CW. Activated caspase-3 expression in Alzheimer’s and aged control brain: correlation with Alzheimer pathology. Brain Res. 2001;898:350–7.
Rohn TT, Head E, Su JH, Anderson AJ, Bahr BA, Cotman CW, et al. Correlation between caspase activation and neurofibrillary tangle formation in Alzheimer’s disease. Am J Pathol. 2001;158:189–98.
Ramalho RM, Viana RJ, Castro RE, Steer CJ, Low WC, Rodrigues CM. Apoptosis in transgenic mice expressing the P301L mutated form of human tau. Mol Med. 2008;14:309–17.
Britti E, Ros J, Esteras N, Abramov AY. Tau inhibits mitochondrial calcium efflux and makes neurons vulnerable to calcium-induced cell death. Cell Calcium. 2020;86:102150.
Zhao Z, Ho L, Suh J, Qin W, Pyo H, Pompl P, et al. A role of P301L tau mutant in anti-apoptotic gene expression, cell cycle and apoptosis. Mol Cell Neurosci. 2003;24:367–79.
Justin Thenmozhi A, Dhivyabharathi M, Manivasagam T, Essa MM. Tannoid principles of Emblica officinalis attenuated aluminum chloride induced apoptosis by suppressing oxidative stress and tau pathology via Akt/GSK-3βsignaling pathway. J Ethnopharmacol. 2016;194:20–29.
Ahmad Rather M, Justin-Thenmozhi A, Manivasagam T, Saravanababu C, Guillemin GJ, Essa MM. Asiatic acid attenuated aluminum chloride-induced Tau pathology, oxidative stress and apoptosis via AKT/GSK-3β signaling pathway in Wistar rats. Neurotox Res. 2019;35:955–68.
Kang Q, Xiang Y, Li D, Liang J, Zhang X, Zhou F, et al. MiR-124-3p attenuates hyperphosphorylation of Tau protein-induced apoptosis via caveolin-1-PI3K/Akt/GSK3β pathway in N2a/APP695swe cells. Oncotarget. 2017;8:24314–26.
Zhang SG, Wang XS, Zhang YD, Di Q, Shi JP, Qian M, et al. Indirubin-3’-monoxime suppresses amyloid-beta-induced apoptosis by inhibiting tau hyperphosphorylation. Neural Regen Res. 2016;11:988–93.
Martin L, Page G, Terro F. Tau phosphorylation and neuronal apoptosis induced by the blockade of PP2A preferentially involve GSK3β. Neurochem Int. 2011;59:235–50.
Tao Q, Wang X, Liu L, Ji Y, Luo Q, Du J, et al. Toxoplasma gondii Chinese I genotype Wh6 strain infection induces tau phosphorylation via activating GSK3β and causes hippocampal neuron apoptosis. Acta Tropica. 2020;210:105560.
Xiao S, Wu Q, Yao X, Zhang J, Zhong W, Zhao J, et al. Inhibitory effects of isobavachalcone on Tau protein aggregation, Tau phosphorylation, and oligomeric Tau-induced apoptosis. ACS Chem Neurosci. 2021;12:123–32.
Xiao N, Zhang F, Zhu B, Liu C, Lin Z, Wang H, et al. CDK5-mediated tau accumulation triggers methamphetamine-induced neuronal apoptosis via endoplasmic reticulum-associated degradation pathway. Toxicol Lett. 2018;292:97–107.
Nie CL, Wang XS, Liu Y, Perrett S, He RQ. Amyloid-like aggregates of neuronal Tau induced by formaldehyde promote apoptosis of neuronal cells. BMC Neurosci. 2007;8:9.
Sola M, Magrin C, Pedrioli G, Pinton S, Salvadè A, Papin S, et al. Tau affects P53 function and cell fate during the DNA damage response. Commun Biol. 2020;3:245.
Liu M, Sui D, Dexheimer T, Hovde S, Deng X, Wang KW, et al. Hyperphosphorylation renders Tau prone to aggregate and to cause cell death. Mol Neurobiol. 2020;57:4704–19.
Lin CI, Chang YC, Kao NJ, Lee WJ, Cross TW, Lin SH. 1,25(OH)(2)D(3) alleviates Aβ(25-35)-induced Tau hyperphosphorylation, excessive reactive oxygen species, and apoptosis through interplay with glial cell line-derived neurotrophic factor signaling in SH-SY5Y cells. Int J Mol Sci. 2020;21:4215.
Sun W, Samimi H, Gamez M, Zare H, Frost B. Pathogenic tau-induced piRNA depletion promotes neuronal death through transposable element dysregulation in neurodegenerative tauopathies. Nat Neurosci. 2018;21:1038–48.
Guo C, Jeong HH, Hsieh YC, Klein HU, Bennett DA, De Jager PL, et al. Tau activates transposable elements in Alzheimer’s Disease. Cell Rep. 2018;23:2874–80.
Wang HH, Li HL, Liu R, Zhang Y, Liao K, Wang Q, et al. Tau overexpression inhibits cell apoptosis with the mechanisms involving multiple viability-related factors. J Alzheimers Dis JAD. 2010;21:167–79.
Wang ZF, Yin J, Zhang Y, Zhu LQ, Tian Q, Wang XC, et al. Overexpression of tau proteins antagonizes amyloid-beta-potentiated apoptosis through mitochondria-caspase-3 pathway in N2a cells. J Alzheimers Dis JAD. 2010;20:145–57.
Liu XA, Song J, Jiang Q, Wang Q, Tian Q, Wang JZ. Expression of the hyperphosphorylated tau attenuates ER stress-induced apoptosis with upregulation of unfolded protein response. Apoptosis Int J Program Cell Death. 2012;17:1039–49.
He A, Zhang C, Ke X, Yi Y, Yu Q, Zhang T, et al. VGLUT3 neurons in median raphe control the efficacy of spatial memory retrieval via ETV4 regulation of VGLUT3 transcription. Sci China Life Sci. 2022;65:1590–607.
Allen B, Ingram E, Takao M, Smith MJ, Jakes R, Virdee K, et al. Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J Neurosci Off J Soc Neurosci. 2002;22:9340–51.
Li HL, Wang HH, Liu SJ, Deng YQ, Zhang YJ, Tian Q, et al. Phosphorylation of tau antagonizes apoptosis by stabilizing beta-catenin, a mechanism involved in Alzheimer’s neurodegeneration. Proc Natl Acad Sci USA. 2007;104:3591–6.
Liu E, Zhou Q, Xie AJ, Li X, Li M, Ye J, et al. Tau acetylates and stabilizes β-catenin thereby promoting cell survival. EMBO Rep. 2020;21:e48328.
Liu XA, Liao K, Liu R, Wang HH, Zhang Y, Zhang Q, et al. Tau dephosphorylation potentiates apoptosis by mechanisms involving a failed dephosphorylation/activation of Bcl-2. J Alzheimer’s Dis JAD. 2010;19:953–62.
Luo DJ, Feng Q, Wang ZH, Sun DS, Wang Q, Wang JZ, et al. Knockdown of phosphotyrosyl phosphatase activator induces apoptosis via mitochondrial pathway and the attenuation by simultaneous tau hyperphosphorylation. J Neurochem. 2014;130:816–25.
de Calignon A, Fox LM, Pitstick R, Carlson GA, Bacskai BJ, Spires-Jones TL, et al. Caspase activation precedes and leads to tangles. Nature. 2010;464:1201–4.
Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–9.
Grootjans S, Vanden Berghe T, Vandenabeele P. Initiation and execution mechanisms of necroptosis: an overview. Cell Death Differ. 2017;24:1184–95.
Tummers B, Green DR. Caspase-8: regulating life and death. Immunol Rev. 2017;277:76–89.
Zhang J, Liu D, Fu P, Liu ZQ, Lai C, Yang CQ, et al. Social isolation reinforces aging-related behavioral inflexibility by promoting neuronal necroptosis in basolateral amygdala. Mol Psychiatry. 2022;27:4050–63.
Yuan J, Amin P, Ofengeim D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev Neurosci. 2019;20:19–33.
Zhu S, Zhang Y, Bai G, Li H. Necrostatin-1 ameliorates symptoms in R6/2 transgenic mouse model of Huntington’s disease. Cell Death Dis. 2011;2:e115.
Re DB, Le Verche V, Yu C, Amoroso MW, Politi KA, Phani S, et al. Necroptosis drives motor neuron death in models of both sporadic and familial ALS. Neuron. 2014;81:1001–8.
Ito Y, Ofengeim D, Najafov A, Das S, Saberi S, Li Y, et al. RIPK1 mediates axonal degeneration by promoting inflammation and necroptosis in ALS. Science. 2016;353:603–8.
Yang SH, Lee DK, Shin J, Lee S, Baek S, Kim J, et al. Nec-1 alleviates cognitive impairment with reduction of Aβ and tau abnormalities in APP/PS1 mice. EMBO Mol Med. 2017;9:61–77.
Jayaraman A, Htike TT, James R, Picon C, Reynolds R. TNF-mediated neuroinflammation is linked to neuronal necroptosis in Alzheimer’s disease hippocampus. Acta Neuropathol Commun. 2021;9:159.
Wu JR, Wang J, Zhou SK, Yang L, Yin JL, Cao JP, et al. Necrostatin-1 protection of dopaminergic neurons. Neural Regen Res. 2015;10:1120–4.
Caccamo A, Branca C, Piras IS, Ferreira E, Huentelman MJ, Liang WS, et al. Necroptosis activation in Alzheimer’s disease. Nat Neurosci. 2017;20:1236–46.
Dong Y, Yu H, Li X, Bian K, Zheng Y, Dai M, et al. Hyperphosphorylated tau mediates neuronal death by inducing necroptosis and inflammation in Alzheimer’s disease. J Neuroinflammation. 2022;19:205.
Koper MJ, Van Schoor E, Ospitalieri S, Vandenberghe R, Vandenbulcke M, von Arnim CAF, et al. Necrosome complex detected in granulovacuolar degeneration is associated with neuronal loss in Alzheimer’s disease. Acta Neuropathol. 2020;139:463–84.
Wiersma VI, van Ziel AM, Vazquez-Sanchez S, Nölle A, Berenjeno-Correa E, Bonaterra-Pastra A, et al. Granulovacuolar degeneration bodies are neuron-selective lysosomal structures induced by intracellular tau pathology. Acta Neuropathol. 2019;138:943–70.
Bondareff W, Wischik CM, Novak M, Roth M. Sequestration of tau by granulovacuolar degeneration in Alzheimer’s disease. Am J Pathol. 1991;139:641–7.
Puladi B, Dinekov M, Arzberger T, Taubert M, Köhler C. The relation between tau pathology and granulovacuolar degeneration of neurons. Neurobiol Dis. 2021;147:105138.
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72.
Dixon SJ. Ferroptosis: bug or feature? Immunol Rev. 2017;277:150–7.
Sripetchwandee J, Pipatpiboon N, Chattipakorn N, Chattipakorn S. Combined therapy of iron chelator and antioxidant completely restores brain dysfunction induced by iron toxicity. PloS One. 2014;9:e85115.
Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med. 2012;18:291–5.
Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci USA. 1997;94:9866–8.
Oakley AE, Collingwood JF, Dobson J, Love G, Perrott HR, Edwardson JA, et al. Individual dopaminergic neurons show raised iron levels in Parkinson disease. Neurology. 2007;68:1820–5.
Yamamoto A, Shin RW, Hasegawa K, Naiki H, Sato H, Yoshimasu F, et al. Iron (III) induces aggregation of hyperphosphorylated tau and its reduction to iron (II) reverses the aggregation: implications in the formation of neurofibrillary tangles of Alzheimer’s disease. J Neurochem. 2002;82:1137–47.
Xie L, Zheng W, Xin N, Xie JW, Wang T, Wang ZY. Ebselen inhibits iron-induced tau phosphorylation by attenuating DMT1 up-regulation and cellular iron uptake. Neurochem Int. 2012;61:334–40.
Guo C, Wang P, Zhong ML, Wang T, Huang XS, Li JY, et al. Deferoxamine inhibits iron induced hippocampal tau phosphorylation in the Alzheimer transgenic mouse brain. Neurochem Int. 2013;62:165–72.
Wan W, Cao L, Kalionis B, Murthi P, Xia S, Guan Y. Iron deposition leads to hyperphosphorylation of Tau and disruption of insulin signaling. Front Neurol. 2019;10:607.
Zhang YH, Wang DW, Xu SF, Zhang S, Fan YG, Yang YY, et al. α-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice. Redox Biol. 2018;14:535–48.
Bao WD, Pang P, Zhou XT, Hu F, Xiong W, Chen K, et al. Loss of ferroportin induces memory impairment by promoting ferroptosis in Alzheimer’s disease. Cell Death Differ. 2021;28:1548–62.
Bao WD, Zhou XT, Zhou LT, Wang F, Yin X, Lu Y, et al. Targeting miR-124/Ferroportin signaling ameliorated neuronal cell death through inhibiting apoptosis and ferroptosis in aged intracerebral hemorrhage murine model. Aging Cell. 2020;19:e13235.
Wang S, Jiang Y, Liu Y, Liu Q, Sun H, Mei M, et al. Ferroptosis promotes microtubule-associated protein tau aggregation via GSK-3β activation and proteasome inhibition. Mol Neurobiol. 2022;59:1486–501.
Chen K, Jiang X, Wu M, Cao X, Bao W, Zhu LQ. Ferroptosis, a potential therapeutic target in Alzheimer’s disease. Front Cell Dev Biol. 2021;9:704298.
Lei P, Ayton S, Appukuttan AT, Moon S, Duce JA, Volitakis I, et al. Lithium suppression of tau induces brain iron accumulation and neurodegeneration. Mol Psychiatry. 2017;22:396–406.
Li X, Lei P, Tuo Q, Ayton S, Li QX, Moon S, et al. Enduring elevations of hippocampal amyloid precursor protein and iron are features of β-amyloid toxicity and are mediated by Tau. NeuroTherapeutics J Am Soc Exp NeuroTherapeutics. 2015;12:862–73.
Tuo QZ, Lei P, Jackman KA, Li XL, Xiong H, Li XL, et al. Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Mol Psychiatry. 2017;22:1520–30.
Lei P, Ayton S, Appukuttan AT, Volitakis I, Adlard PA, Finkelstein DI, et al. Clioquinol rescues Parkinsonism and dementia phenotypes of the tau knockout mouse. Neurobiol Dis. 2015;81:168–75.
Berger GE, Wood SJ, Ross M, Hamer CA, Wellard RM, Pell G, et al. Neuroprotective effects of low-dose lithium in individuals at ultra-high risk for psychosis. a longitudinal MRI/MRS study. Curr Pharm Des. 2012;18:570–5.
Rametti A, Esclaire F, Yardin C, Cogné N, Terro F. Lithium down-regulates tau in cultured cortical neurons: a possible mechanism of neuroprotection. Neurosci Lett. 2008;434:93–98.
Bi M, Gladbach A, van Eersel J, Ittner A, Przybyla M, van Hummel A, et al. Tau exacerbates excitotoxic brain damage in an animal model of stroke. Nat Commun. 2017;8:473.
Chen W, Jiang L, Hu Y, Tang N, Liang N, Li XF, et al. Ferritin reduction is essential for cerebral ischemia-induced hippocampal neuronal death through p53/SLC7A11-mediated ferroptosis. Brain Res. 2021;1752:147216.
Pei L, Wang S, Jin H, Bi L, Wei N, Yan H, et al. A novel mechanism of spine damages in stroke via DAPK1 and Tau. Cereb Cortex. 2015;25:4559–71.
Chen YD, Huang PY, Chiang CS, Huang YS, Tang SC. Generation and role of calpain-cleaved 17-kDa Tau fragment in acute ischemic stroke. Mol Neurobiol. 2021;58:5814–25.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5.
Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–6.
Voet S, Srinivasan S, Lamkanfi M, van Loo G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol Med. 2019;11:e10248.
Ising C, Venegas C, Zhang S, Scheiblich H, Schmidt SV, Vieira-Saecker A, et al. NLRP3 inflammasome activation drives tau pathology. Nature. 2019;575:669–73.
Shen H, Han C, Yang Y, Guo L, Sheng Y, Wang J, et al. Pyroptosis executive protein GSDMD as a biomarker for diagnosis and identification of Alzheimer’s disease. Brain Behav. 2021;11:e02063.
Li Y, Xu P, Shan J, Sun W, Ji X, Chi T, et al. Interaction between hyperphosphorylated tau and pyroptosis in forskolin and streptozotocin induced AD models. Biomed Pharmacother. 2020;121:109618.
Sui G, Yang C, Wang L, Xiong X, Guo M, Chen Z, et al. Exogenous IGF-1 improves tau pathology and neuronal pyroptosis in high-fat diet mice with cognitive dysfunction. Metab Brain Dis. 2021;36:2079–88.
Stancu IC, Cremers N, Vanrusselt H, Couturier J, Vanoosthuyse A, Kessels S, et al. Aggregated Tau activates NLRP3-ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathol. 2019;137:599–617.
Heneka MT, McManus RM, Latz E. Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci. 2018;19:610–21.
Tian D, Xing Y, Gao W, Zhang H, Song Y, Tian Y, et al. Sevoflurane aggravates the progress of Alzheimer’s disease through NLRP3/Caspase-1/Gasdermin D pathway. Front Cell Dev Biol. 2021;9:801422.
Panda C, Voelz C, Habib P, Mevissen C, Pufe T, Beyer C, et al. Aggregated Tau-PHF6 (VQIVYK) potentiates NLRP3 inflammasome expression and autophagy in human microglial cells. Cells. 2021;10:1652.
Cai Y, Chai Y, Fu Y, Wang Y, Zhang Y, Zhang X, et al. Salidroside ameliorates Alzheimer’s disease by targeting NLRP3 inflammasome-mediated pyroptosis. Front Aging Neurosci. 2021;13:809433.
Liu Y, Levine B. Autosis and autophagic cell death: the dark side of autophagy. Cell Death Differ. 2015;22:367–76.
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–20.
Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–10.
Uchiyama Y, Koike M, Shibata M. Autophagic neuron death in neonatal brain ischemia/hypoxia. Autophagy. 2008;4:404–8.
Shi R, Weng J, Zhao L, Li XM, Gao TM, Kong J. Excessive autophagy contributes to neuron death in cerebral ischemia. CNS Neurosci Therapeutics. 2012;18:250–60.
Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–41.
Berry DL, Baehrecke EH. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell. 2007;131:1137–48.
Denton D, Shravage B, Simin R, Mills K, Berry DL, Baehrecke EH, et al. Autophagy, not apoptosis, is essential for midgut cell death in Drosophila. Curr Biol. 2009;19:1741–6.
Nixon RA, Yang DS. Autophagy and neuronal cell death in neurological disorders. Cold Spring Harb Perspect Biol. 2012;4:a008839.
Shimizu S, Konishi A, Nishida Y, Mizuta T, Nishina H, Yamamoto A, et al. Involvement of JNK in the regulation of autophagic cell death. Oncogene. 2010;29:2070–82.
Shimizu S, Kanaseki T, Mizushima N, Mizuta T, Arakawa-Kobayashi S, Thompson CB, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol. 2004;6:1221–8.
Yu L, Wan F, Dutta S, Welsh S, Liu Z, Freundt E, et al. Autophagic programmed cell death by selective catalase degradation. Proc Natl Acad Sci USA. 2006;103:4952–7.
Lamy L, Ngo VN, Emre NC, Shaffer AL 3rd, Yang Y, Tian E, et al. Control of autophagic cell death by caspase-10 in multiple myeloma. Cancer Cell. 2013;23:435–49.
Qiu L, Ng G, Tan EK, Liao P, Kandiah N, Zeng L. Chronic cerebral hypoperfusion enhances Tau hyperphosphorylation and reduces autophagy in Alzheimer’s disease mice. Sci Rep. 2016;6:23964.
Xu Y, Propson NE, Du S, Xiong W, Zheng H. Autophagy deficiency modulates microglial lipid homeostasis and aggravates tau pathology and spreading. Proc Natl Acad Sci USA. 2021;118:e2023418118.
Ji C, Tang M, Zeidler C, Höhfeld J, Johnson GV. BAG3 and SYNPO (synaptopodin) facilitate phospho-MAPT/Tau degradation via autophagy in neuronal processes. Autophagy. 2019;15:1199–213.
Jo C, Gundemir S, Pritchard S, Jin YN, Rahman I, Johnson GV. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat Commun. 2014;5:3496.
Chambraud B, Daguinot C, Guillemeau K, Genet M, Dounane O, Meduri G, et al. Decrease of neuronal FKBP4/FKBP52 modulates perinuclear lysosomal positioning and MAPT/Tau behavior during MAPT/Tau-induced proteotoxic stress. Autophagy. 2021;17:3491–510.
Meduri G, Guillemeau K, Dounane O, Sazdovitch V, Duyckaerts C, Chambraud B, et al. Caspase-cleaved Tau-D(421) is colocalized with the immunophilin FKBP52 in the autophagy-endolysosomal system of Alzheimer’s disease neurons. Neurobiol Aging. 2016;46:124–37.
Blair LJ, Nordhues BA, Hill SE, Scaglione KM, O’Leary JC 3rd, Fontaine SN, et al. Accelerated neurodegeneration through chaperone-mediated oligomerization of tau. J Clin Investig. 2013;123:4158–69.
Gassen NC, Hartmann J, Zannas AS, Kretzschmar A, Zschocke J, Maccarrone G, et al. FKBP51 inhibits GSK3β and augments the effects of distinct psychotropic medications. Mol Psychiatry. 2016;21:277–89.
Caballero B, Bourdenx M, Luengo E, Diaz A, Sohn PD, Chen X, et al. Acetylated tau inhibits chaperone-mediated autophagy and promotes tau pathology propagation in mice. Nat Commun. 2021;12:2238.
Xilouri M, Vogiatzi T, Vekrellis K, Park D, Stefanis L. Abberant alpha-synuclein confers toxicity to neurons in part through inhibition of chaperone-mediated autophagy. PloS One. 2009;4:e5515.
Dai B, Zhong T, Chen ZX, Chen W, Zhang N, Liu XL, et al. Myricetin slows liquid-liquid phase separation of Tau and activates ATG5-dependent autophagy to suppress Tau toxicity. J Biol Chem. 2021;297:101222.
Yang Z, Zhou C, Shi H, Zhang N, Tang B, Ji N. Heme induces BECN1/ATG5-mediated autophagic cell death via ER stress in neurons. Neurotox Res. 2020;38:1037–48.
Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–2.
Choi GE, Lee HJ, Chae CW, Cho JH, Jung YH, Kim JS, et al. BNIP3L/NIX-mediated mitophagy protects against glucocorticoid-induced synapse defects. Nat Commun. 2021;12:487.
Martinelli S, Anderzhanova EA, Bajaj T, Wiechmann S, Dethloff F, Weckmann K, et al. Stress-primed secretory autophagy promotes extracellular BDNF maturation by enhancing MMP9 secretion. Nat Commun. 2021;12:4643.
Silva JM, Rodrigues S, Sampaio-Marques B, Gomes P, Neves-Carvalho A, Dioli C, et al. Dysregulation of autophagy and stress granule-related proteins in stress-driven Tau pathology. Cell Death Differ. 2019;26:1411–27.
Feng Q, Luo Y, Zhang XN, Yang XF, Hong XY, Sun DS, et al. MAPT/Tau accumulation represses autophagy flux by disrupting IST1-regulated ESCRT-III complex formation: a vicious cycle in Alzheimer neurodegeneration. Autophagy. 2020;16:641–58.
Bowles KR, Silva MC, Whitney K, Bertucci T, Berlind JE, Lai JD, et al. ELAVL4, splicing, and glutamatergic dysfunction precede neuron loss in MAPT mutation cerebral organoids. Cell. 2021;184:4547–63.e4517.
Zhang J, Cai T, Zhao F, Yao T, Chen Y, Liu X, et al. The role of α-synuclein and tau hyperphosphorylation-mediated autophagy and apoptosis in lead-induced learning and memory injury. Int J Biol Sci. 2012;8:935–44.
Mann CN, Devi SS, Kersting CT, Bleem AV, Karch CM, Holtzman DM, et al. Astrocytic α2-Na(+)/K(+) ATPase inhibition suppresses astrocyte reactivity and reduces neurodegeneration in a tauopathy mouse model. Sci Transl Med. 2022;14:eabm4107.
Kurematsu C, Sawada M, Ohmuraya M, Tanaka M, Kuboyama K, Ogino T, et al. Synaptic pruning of murine adult-born neurons by microglia depends on phosphatidylserine. J Exp Med. 2022;219:e20202304.
Gardai SJ, McPhillips KA, Frasch SC, Janssen WJ, Starefeldt A, Murphy-Ullrich JE, et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell. 2005;123:321–34.
Scott-Hewitt N, Perrucci F, Morini R, Erreni M, Mahoney M, Witkowska A, et al. Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J. 2020;39:e105380.
Butler CA, Popescu AS, Kitchener EJA, Allendorf DH, Puigdellívol M, Brown GC. Microglial phagocytosis of neurons in neurodegeneration, and its regulation. J Neurochem. 2021;158:621–39.
Païdassi H, Tacnet-Delorme P, Garlatti V, Darnault C, Ghebrehiwet B, Gaboriaud C, et al. C1q binds phosphatidylserine and likely acts as a multiligand-bridging molecule in apoptotic cell recognition. J Immunol. 2008;180:2329–38.
Brown GC, Neher JJ. Microglial phagocytosis of live neurons. Nat Rev Neurosci. 2014;15:209–16.
Podleśny-Drabiniok A, Marcora E, Goate AM. Microglial phagocytosis: a disease-associated process emerging from Alzheimer’s disease genetics. Trends Neurosci. 2020;43:965–79.
Bellucci A, Westwood AJ, Ingram E, Casamenti F, Goedert M, Spillantini MG. Induction of inflammatory mediators and microglial activation in mice transgenic for mutant human P301S tau protein. Am J Pathol. 2004;165:1643–52.
Brelstaff J, Tolkovsky AM, Ghetti B, Goedert M, Spillantini MG. Living neurons with tau filaments aberrantly expose phosphatidylserine and are phagocytosed by microglia. Cell Rep. 2018;24:1939–48.e1934.
Benetatos J, Bennett RE, Evans HT, Ellis SA, Hyman BT, Bodea LG, et al. PTEN activation contributes to neuronal and synaptic engulfment by microglia in tauopathy. Acta Neuropathol. 2020;140:7–24.
Brelstaff JH, Mason M, Katsinelos T, McEwan WA, Ghetti B, Tolkovsky AM, et al. Microglia become hypofunctional and release metalloproteases and tau seeds when phagocytosing live neurons with P301S tau aggregates. Sci Adv. 2021;7:eabg4980.
Hopp SC, Lin Y, Oakley D, Roe AD, DeVos SL, Hanlon D, et al. The role of microglia in processing and spreading of bioactive tau seeds in Alzheimer’s disease. J Neuroinflammation. 2018;15:269.
Pampuscenko K, Morkuniene R, Sneideris T, Smirnovas V, Budvytyte R, Valincius G, et al. Extracellular tau induces microglial phagocytosis of living neurons in cell cultures. J Neurochem. 2020;154:316–29.
Das R, Balmik AA, Chinnathambi S. Phagocytosis of full-length Tau oligomers by Actin-remodeling of activated microglia. J Neuroinflammation. 2020;17:10.
Wu T, Dejanovic B, Gandham VD, Gogineni A, Edmonds R, Schauer S, et al. Complement C3 is activated in human AD brain and is required for neurodegeneration in mouse models of amyloidosis and tauopathy. Cell Rep. 2019;28:2111–23.e2116.
Litvinchuk A, Wan YW, Swartzlander DB, Chen F, Cole A, Propson NE, et al. Complement C3aR inactivation attenuates Tau pathology and reverses an immune network deregulated in tauopathy models and Alzheimer’s disease. Neuron. 2018;100:1337–53.e1335.
Dejanovic B, Huntley MA, De Mazière A, Meilandt WJ, Wu T, Srinivasan K, et al. Changes in the synaptic proteome in tauopathy and rescue of Tau-induced synapse loss by C1q antibodies. Neuron. 2018;100:1322–36.e1327.
Luo W, Liu W, Hu X, Hanna M, Caravaca A, Paul SM. Microglial internalization and degradation of pathological tau is enhanced by an anti-tau monoclonal antibody. Sci Rep. 2015;5:11161.
Desale SE, Chinnathambi S. α- Linolenic acid modulates phagocytosis and endosomal pathways of extracellular Tau in microglia. Cell Adhes Migr. 2021;15:84–100.
Funding
This study was partially supported by the National Key Research and Development Program of China (2019YFE0121200 to LQZ), the National Natural Science Foundation of China (82030032 to LQZ, 82261138555, 32070960 to DL, 82260209 and 81960221 to XPY, and 82260454 to BB), Top-Notch Young Talents Program of China of 2014 and Academic Frontier Youth Team of Huazhong University of Science and Technology (to LQZ); the Hubei Provincial Natural Science Foundation (2022CFA004 to LQ); the National Science & Technology Fundamental Resource Investigation Program of China (2018FY100903 to XPY); and Science and Technology Project Founded by the Education Department of Jiangxi Province (GJJ201834 to MXW). Jiangxi Provincial Health Commission Science and Technology Plan Project (202212021 to MXW and 202311506 to ZYC), and Jiangxi Provincial Administration of Traditional Chinese Medicine Science and Technology Plan Project (2022A322 to ZYC).
Author information
Authors and Affiliations
Contributions
The initial idea for this review was generated by LQZ and, DL, and the manuscript was written and revised by MXW, ZYC and MJ. In addition, MXW, MJ and BB contributed to illustration preparation; LQZ, XPY, DLL and ZYC edited the manuscript; DL, XRW, XPY and MXW read, reviewed, and approved the final manuscript. The final version of the manuscript was approved by all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, M., Chen, Z., Jiang, M. et al. Friend or foe: role of pathological tau in neuronal death. Mol Psychiatry 28, 2215–2227 (2023). https://doi.org/10.1038/s41380-023-02024-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-02024-z
This article is cited by
-
Silencing tau to treat early Alzheimer’s disease
Nature Medicine (2023)
-
Neuronal tau pathology worsens late-phase white matter degeneration after traumatic brain injury in transgenic mice
Acta Neuropathologica (2023)