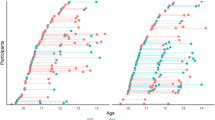
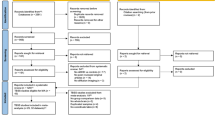
Abstract
The neurodevelopmental model of schizophrenia is supported by multi-level impairments shared among schizophrenia and neurodevelopmental disorders. Despite schizophrenia and typical neurodevelopmental disorders, i.e., autism spectrum disorder (ASD) and attention-deficit/hyperactivity disorder (ADHD), as disorders of brain dysconnectivity, no study has ever elucidated whether whole-brain white matter (WM) tracts integrity alterations overlap or diverge between these three disorders. Moreover, whether the linked dimensions of cognition and brain metrics per the Research Domain Criteria framework cut across diagnostic boundaries remains unknown. We aimed to map deviations from normative ranges of whole-brain major WM tracts for individual patients to investigate the similarity and differences among schizophrenia (281 patients subgrouped into the first-episode, subchronic and chronic phases), ASD (175 patients), and ADHD (279 patients). Sex-specific WM tract normative development was modeled from diffusion spectrum imaging of 626 typically developing controls (5–40 years). There were three significant findings. First, the patterns of deviation and idiosyncrasy of WM tracts were similar between schizophrenia and ADHD alongside ASD, particularly at the earlier stages of schizophrenia relative to chronic stages. Second, using the WM deviation patterns as features, schizophrenia cannot be separated from neurodevelopmental disorders in the unsupervised machine learning algorithm. Lastly, the canonical correlation analysis showed schizophrenia, ADHD, and ASD shared linked cognitive dimensions driven by WM deviations. Together, our results provide new insights into the neurodevelopmental facet of schizophrenia and its brain basis. Individual’s WM deviations may contribute to diverse arrays of cognitive function along a continuum with phenotypic expressions from typical neurodevelopmental disorders to schizophrenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD, et al. Schizophrenia. Nat Rev Dis Prim. 2015;1:15067.
Birnbaum R, Weinberger DR. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat Rev Neurosci. 2017;18:727–40.
Pantelis C, Yücel M, Wood SJ, Velakoulis D, Sun D, Berger G, et al. Structural brain imaging evidence for multiple pathological processes at different stages of brain development in schizophrenia. Schizophrenia Bull. 2005;31:672–96.
Kochunov P, Hong LE. Neurodevelopmental and neurodegenerative models of schizophrenia: white matter at the center stage. Schizophr Bull. 2014;40:721–8.
Oliver LD, Moxon-Emre I, Lai MC, Grennan L, Voineskos AN, Ameis SH. Social Cognitive Performance in Schizophrenia Spectrum Disorders Compared With Autism Spectrum Disorder: A Systematic Review, Meta-analysis, and Meta-regression. JAMA Psychiatry. 2021;78:281–92.
Arican I, Bass N, Neelam K, Wolfe K, McQuillin A, Giaroli G. Prevalence of attention deficit hyperactivity disorder symptoms in patients with schizophrenia. Acta Psychiatr Scand. 2019;139:89–96.
Keshavan MS, Diwadkar VA, Montrose DM, Rajarethinam R, Sweeney JA. Premorbid indicators and risk for schizophrenia: a selective review and update. Schizophrenia Res. 2005;79:45–57.
Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Prim. 2015;1:15020.
Lord C, Brugha TS, Charman T, Cusack J, Dumas G, Frazier T, et al. Autism spectrum disorder. Nat Rev Dis Prim. 2020;6:5.
Brainstorm C, Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, et al. Analysis of shared heritability in common disorders of the brain. Science. 2018;360:6395.
Gudmundsson OO, Walters GB, Ingason A, Johansson S, Zayats T, Athanasiu L, et al. Attention-deficit hyperactivity disorder shares copy number variant risk with schizophrenia and autism spectrum disorder. Transl Psychiatry. 2019;9:258.
Spronk M, Keane BP, Ito T, Kulkarni K, Ji JL, Anticevic A et al. A Whole-Brain and Cross-Diagnostic Perspective on Functional Brain Network Dysfunction. Cereb Cortex. 2021;31:547–61.
Waldman ID, Poore HE, Luningham JM, Yang J. Testing structural models of psychopathology at the genomic level. World Psychiatry. 2020;19:350–9.
Opel N, Goltermann J, Hermesdorf M, Berger K, Baune BT, Dannlowski U. Cross-Disorder Analysis of Brain Structural Abnormalities in Six Major Psychiatric Disorders: A Secondary Analysis of Mega- and Meta-analytical Findings From the ENIGMA Consortium. Biol Psychiatry. 2020;88:678–86.
Marquand AF, Kia SM, Zabihi M, Wolfers T, Buitelaar JK, Beckmann CF. Conceptualizing mental disorders as deviations from normative functioning. Mol psychiatry. 2019;24:1415–24.
Lv J, Di Biase M, Cash RFH, Cocchi L, Cropley VL, Klauser P et al. Individual deviations from normative models of brain structure in a large cross-sectional schizophrenia cohort. Mol Psychiatry. 2021;26:3512–23.
Tung YH, Lin HY, Chen CL, Shang CY, Yang LY, Hsu YC et al. Whole-brain white matter tracts deviation and idiosyncrasy from normative development in autism, ADHD and their unaffected siblings link with dimensions of psychopathology and cognition. Am J Psychiatry. 2021;178:730–43.
Honey CJ, Kotter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity on multiple time scales. Proc Natl Acad Sci USA. 2007;104:10240–5.
Alexander-Bloch A, Giedd JN, Bullmore E. Imaging structural co-variance between human brain regions. Nat Rev Neurosci. 2013;14:322–36.
Kochunov P, Hong LE, Dennis EL, Morey RA, Tate DF, Wilde EA et al. ENIGMA-DTI: Translating reproducible white matter deficits into personalized vulnerability metrics in cross-diagnostic psychiatric research. Hum Brain Mapp. 2022;43:194–206.
Salthouse TA. Aging and measures of processing speed. Biol Psychol. 2000;54:35–54.
Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, et al. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. 2010;31:1554–62.
Kochunov P, Coyle T, Lancaster J, Robin DA, Hardies J, Kochunov V, et al. Processing speed is correlated with cerebral health markers in the frontal lobes as quantified by neuroimaging. Neuroimage. 2010;49:1190–9.
Penke L, Muñoz Maniega S, Murray C, Gow AJ, Hernández MC, Clayden JD, et al. A general factor of brain white matter integrity predicts information processing speed in healthy older people. J Neurosci. 2010;30:7569–74.
Wright SN, Hong LE, Winkler AM, Chiappelli J, Nugent K, Muellerklein F, et al. Perfusion shift from white to gray matter may account for processing speed deficits in schizophrenia. Hum Brain Mapp. 2015;36:3793–804.
Pérez-Iglesias R, Tordesillas-Gutiérrez D, McGuire PK, Barker GJ, Roiz-Santiañez R, Mata I, et al. White matter integrity and cognitive impairment in first-episode psychosis. Am J Psychiatry. 2010;167:451–8.
Glahn DC, Kent JW Jr., Sprooten E, Diego VP, Winkler AM, Curran JE, et al. Genetic basis of neurocognitive decline and reduced white-matter integrity in normal human brain aging. Proc Natl Acad Sci USA. 2013;110:19006–11.
Karbasforoushan H, Duffy B, Blackford JU, Woodward ND. Processing speed impairment in schizophrenia is mediated by white matter integrity. Psychol Med. 2015;45:109–20.
Turken A, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage. 2008;42:1032–44.
Alloza C, Cox SR, Duff B, Semple SI, Bastin ME, Whalley HC, et al. Information processing speed mediates the relationship between white matter and general intelligence in schizophrenia. Psychiatry Res Neuroimaging. 2016;254:26–33.
Hong SB, Zalesky A, Fornito A, Park S, Yang YH, Park MH, et al. Connectomic disturbances in attention-deficit/hyperactivity disorder: a whole-brain tractography analysis. Biol Psychiatry. 2014;76:656–63.
Fuelscher I, Hyde C, Anderson V, Silk TJ. White matter tract signatures of fiber density and morphology in ADHD. Cortex. 2021;138:329–40.
Barnea-Goraly N, Lotspeich LJ, Reiss AL. Similar white matter aberrations in children with autism and their unaffected siblings: a diffusion tensor imaging study using tract-based spatial statistics. Arch Gen Psychiatry. 2010;67:1052–60.
Jou RJ, Reed HE, Kaiser MD, Voos AC, Volkmar FR, Pelphrey KA. White Matter Abnormalities in Autism and Unaffected Siblings. J Neuropsychiatry Clin Neurosci. 2016;28:49–55.
Di X, Azeez A, Li X, Haque E, Biswal BB. Disrupted focal white matter integrity in autism spectrum disorder: A voxel-based meta-analysis of diffusion tensor imaging studies. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;82:242–8.
Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, et al. Research Domain Criteria (RDoC): Toward a New Classification Framework for Research on Mental Disorders. Am J Psychiatry. 2010;167:748–51.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. 4th ed edn. Washington DC:American Psychiatric Association;1994.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. 5th ed edn. Arlinton, VA:American Psychiatric Association;2013.
Gau SSF, Chou MC, Lee JC, Wong CC, Chou WJ, Chen MF, et al. Behavioral problems and parenting style among Taiwanese children with autism and their siblings. Psychiatry Clin Neurosci. 2010;64:70–8.
Gau SS, Chong MY, Chen TH, Cheng AT. A 3-year panel study of mental disorders among adolescents in Taiwan. Am J Psychiatry. 2005;162:1344–50.
Chen YL, Shen LJ, Gau SS. The Mandarin version of the Kiddie-Schedule for Affective Disorders and Schizophrenia-Epidemiological version for DSM-5 - A psychometric study. J Formos Med Assoc. 2017;116:671–8. Epub 2017 Jul 1011
Lin YJ, Yang LK, Gau SS. Psychiatric comorbidities of adults with early- and late-onset attention-deficit/hyperactivity disorder. Aust N. Z J Psychiatry. 2016;50:548–56. Epub 0004867415602015 Oct 0004867415609412.
Lin HY, Cocchi L, Zalesky A, Lv J, Perry A, Tseng WI, et al. Brain-behavior patterns define a dimensional biotype in medication-naive adults with attention-deficit hyperactivity disorder. Psychol Med. 2018;48:2399–408. Epub 0033291718002018 Feb 0033291718000027.
Newton R, Rouleau A, Nylander AG, Loze JY, Resemann HK, Steeves S, et al. Diverse definitions of the early course of schizophrenia-a targeted literature review. NPJ Schizophr. 2018;4:21.
Wechsler D. Wechsler Adult Intelligence Scale - Third Edition (WAIS-III). San Antonio, TX:Psychological Corporation;1997.
Wechsler D. Wechsler Intelligence Scale for Children - Third Edition (WISC-III). San Antonio, TX:Psychological Corporation;1991.
Lin YJ, Gau SS. Developmental changes of neuropsychological functioning in individuals with and without childhood ADHD from early adolescence to young adulthood: a 7-year follow-up study. Psychol Med. 2019;49:940–51. Epub 0033291718002018 Jun 0033291718001526.
Luciana M. Practitioner Review: Computerized assessment of neuropsychological function in children: clinical and research applications of the Cambridge Neuropsychological Testing Automated Battery (CANTAB). J Child Psychol Psychiatry. 2003;44:649–63.
Seng GJ, Tseng WL, Chiu YN, Tsai WC, Wu YY, Gau SS. Executive functions in youths with autism spectrum disorder and their unaffected siblings. Psychol Med. 2020;30:1–10.
Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy‐current‐induced distortion in diffusion MRI using a twice‐refocused spin echo. Magn Reson Med. 2002;49:177–82.
Hsu Y-C, Hsu C-H, Tseng, YI W-. A large deformation diffeomorphic metric mapping solution for diffusion spectrum imaging datasets. NeuroImage. 2012;63:818–34.
Yeh F-C, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng W-YI. Deterministic Diffusion Fiber Tracking Improved by Quantitative Anisotropy. PLOS ONE. 2013;8:e80713.
Avram AV, Sarlls JE, Barnett AS, Ozarslan E, Thomas C, Irfanoglu MO, et al. Clinical feasibility of using mean apparent propagator (MAP) MRI to characterize brain tissue microstructure. Neuroimage. 2016;127:422–34.
Snedecor GW, Cochran WG. Statistical Methods. 8th edn. Ames, Iowa:Iowa State University Press;1989.
Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar Behav Res. 2011;46:399–424.
Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a data set via the gap statistic. J R Stat Soc: Ser B (Stat Methodol). 2001;63:411–23.
Smith SM, Nichols TE, Vidaurre D, Winkler AM, Behrens TEJ, Glasser MF, et al. A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci. 2015;18:1565–7.
Alexander AL, Hurley SA, Samsonov AA, Adluru N, Hosseinbor AP, Mossahebi P, et al. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connectivity. 2011;1:423–46.
Rommelse N, Buitelaar JK, Hartman CA. Structural brain imaging correlates of ASD and ADHD across the lifespan: a hypothesis-generating review on developmental ASD-ADHD subtypes. J Neural Transm (Vienna). 2017;124:259–71.
Thapar A, Riglin L. The importance of a developmental perspective in Psychiatry: what do recent genetic-epidemiological findings show? Mol Psychiatry. 2020;25:1631–9.
Gogtay N, Vyas NS, Testa R, Wood SJ, Pantelis C. Age of onset of schizophrenia: perspectives from structural neuroimaging studies. Schizophr Bull. 2011;37:504–13.
McGorry PD, Nelson B, Goldstone S, Yung AR. Clinical staging: a heuristic and practical strategy for new research and better health and social outcomes for psychotic and related mood disorders. Can J Psychiatry. 2010;55:486–97.
Di Biase MA, Cropley VL, Baune BT, Olver J, Amminger GP, Phassouliotis C, et al. White matter connectivity disruptions in early and chronic schizophrenia. Psychol Med. 2017;47:2797–810.
Owen JP, Marco EJ, Desai S, Fourie E, Harris J, Hill SS, et al. Abnormal white matter microstructure in children with sensory processing disorders. Neuroimage Clin. 2013;2:844–53.
Kallankari H, Saunavaara V, Parkkola R, Haataja L, Hallman M, Kaukola T. Diffusion tensor imaging in frontostriatal tracts is associated with executive functioning in very preterm children at 9 years of age. Pediatr Radiol. 2021;51:112–8
Shen KK, Welton T, Lyon M, McCorkindale AN, Sutherland GT, Burnham S, et al. Structural core of the executive control network: A high angular resolution diffusion MRI study. Hum Brain Mapp. 2020;41:1226–36.
Humpston CS, Broome MR. Thinking, believing, and hallucinating self in schizophrenia. Lancet Psychiatry. 2020;7:638–46.
Heinz A, Murray GK, Schlagenhauf F, Sterzer P, Grace AA, Waltz JA. Towards a Unifying Cognitive, Neurophysiological, and Computational Neuroscience Account of Schizophrenia. Schizophr Bull. 2019;45:1092–1100.
Chini M, Hanganu-Opatz IL. Prefrontal Cortex Development in Health and Disease: Lessons from Rodents and Humans. Trends Neurosci. 2021;44:227–40.
Griffa A, Baumann PS, Klauser P, Mullier E, Cleusix M, Jenni R, et al. Brain connectivity alterations in early psychosis: from clinical to neuroimaging staging. Transl Psychiatry. 2019;9:62.
Morris-Rosendahl DJ, Crocq MA. Neurodevelopmental disorders-the history and future of a diagnostic concept. Dialogues Clin Neurosci. 2020;22:65–72.
Acknowledgements
The study was approved by the Research Ethics Committee of National Taiwan University Hospital, Taipei, Taiwan (Approval numbers: 200903062 R, 201003087 R, and 201201006RIB, corresponding to ClinicalTrials.gov number, NCT00916851, NCT01247610, and NCT01582256, respectively). Written informed consent and child assent were obtained from the participants or their parents. This work was supported by grants from the Ministry of Science and Technology, Taiwan (NSC96–3112-B-002–033, NSC97–3112-B-002–009, NSC98–3112-B-002–004, NSC99–2627-B-002–015, NSC100–2627-B-002–014, NSC99–2321-B-002–037, NSC100–2321-B-002–015, NSC101–2627-B-002–002, NSC 101–2314-B-002–136-MY3, NSC101–2321-B-002–079), the National Health Research Institute, Taiwan (NHRI-EX97–9407PC, NHRI-EX98–9407PC, NHRI-EX100–10008PI, NHRI-EX101–10008PI, NHRI-EX102–10008PI, NHRI-EX103–10008PI, NHRI-EX104–10404PI, NHRI-EX105–10404PI, NHRI-EX106–10404PI), National Taiwan University Hospital (NTUH101-S1910) and Chen-Yung Foundation to SSG for the data collection on ADHD, ASD, and TDC. For data collection of schizophrenia, WYT, HGH, and CML obtained grants from the Ministry of Science and Technology (MOST106–2314-B-002–242-MY3, NSC 100–2321-B-002–016 & NSC 99–2321-B-002–038, MOST 104–2314-B-002–068), respectively. HYL receives stipend support from the Azrieli Foundation through the Azrieli Adult Neurodevelopmental Centre at CAMH. The authors are grateful to all the participants and their parents for their participation and research assistants for data collection.
Author information
Authors and Affiliations
Contributions
SSG initiated and designed the study. YLC, HYL, YHT, and SSG formulated overarching research goals and aims and interpreted the data. SSG collected ASD, ADHD (CYS, too), and control samples. CML, HGH, and TJH collected schizophrenia and control samples. YHT, HYL, CLC, and WYT performed neuroimage analysis. YHT, HYL, CSW, and YLC performed statistical analyses. YLC and YHT drafted the first paper, rigorously edited and reviewed by HYL and SSG. All the authors approved the final version to be published and agreed to be accountable for the integrity and accuracy of all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chien, YL., Lin, HY., Tung, YH. et al. Neurodevelopmental model of schizophrenia revisited: similarity in individual deviation and idiosyncrasy from the normative model of whole-brain white matter tracts and shared brain-cognition covariation with ADHD and ASD. Mol Psychiatry 27, 3262–3271 (2022). https://doi.org/10.1038/s41380-022-01636-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01636-1
This article is cited by
-
Schizophrenia endothelial cells exhibit higher permeability and altered angiogenesis patterns in patient-derived organoids
Translational Psychiatry (2024)
-
Sex and gender in neurodevelopmental conditions
Nature Reviews Neurology (2023)