Abstract
Women with schizophrenia and their newborns are at risk of adverse pregnancy, delivery, neonatal and child outcomes. However, robust and informative epidemiological estimates are lacking to guide health policies to prioritise and organise perinatal services. For the first time, we carried out a systematic review and meta-analysis to synthesise the accumulating evidence on pregnancy, delivery, neonatal complications, and infant mortality among women with schizophrenia and their newborns (N = 43,611) vs. controls (N = 40,948,272) between 1999 and 2021 (26 population-based studies from 11 high-income countries) using random effects. Women with schizophrenia had higher odds (OR) of gestational diabetes (2.35, 95% CI: [1.57–3.52]), gestational hypertension, pre-eclampsia/eclampsia (OR 1.55, 95% CI: [1.02–2.36]; 1.85, 95% CI: [1.52–2.25]), antepartum and postpartum haemorrhage (OR 2.28, 95% CI: [1.58–3.29]; 1.14, 95% CI: [1.04–1.24]), placenta abruption, threatened preterm labour, and premature rupture of membrane (OR 2.20, 95% CI: [2.02–2.39]; 2.91, 95% CI: [1.57–5.40]; 1.29, 95% CI: [1.06–1.58]), c-section (OR 1.33, 95% CI: [1.22–1.45]), foetal distress (OR 1.80, 95% CI: [1.43–2.26]), preterm and very preterm delivery (OR 1.79, 95% CI: [1.62–1.98]; 2.31, 95% CI: [1.78–2.98]), small for gestational age and low birth weight (OR 1.63, 95% CI: [1.48–1.80]; 1.75, 95% CI: [1.46–2.11]), congenital malformations (OR 1.86, 95% CI: [1.71–2.03]), and stillbirths (OR 2.06, 95% CI: [1.83–2.31]). Their newborns had higher odds of neonatal death (OR 1.41, 95% CI: [1.03–1.94]), post-neonatal death (OR 2.87, 95% CI: [2.11–3.89]) and infant mortality (OR 2.33, 95% CI: [1.81–3.01]). This large‐scale meta‐analysis confirms that schizophrenia is associated with a substantially increased risk of very preterm delivery, stillbirth, and infant mortality, and metabolic risk in mothers. No population-based study has been carried out in low- and middle-income countries in which health problems of women with schizophrenia are probably more pronounced. More research is needed to better understand the complex needs of women with schizophrenia and their newborns, determine how care delivery could be optimised, and define best practices. Study registration: PROSPERO CRD42020197446.
Similar content being viewed by others
Introduction
Perinatal mental disorders are frequent and are associated with substantial pregnancy, delivery, neonatal and child morbidity, and mortality [1,2,3]. The World Health Organisation (WHO) has highlighted the urgent need for ‘evidence-based and human rights-oriented mental health care services for early identification and management of maternal mental disorders’ [4]. Women with schizophrenia represent an extremely vulnerable and underserved population [5, 6] who have been neglected in perinatal health services research [5]. Schizophrenia is a chronic and severe mental disorder affecting 20 million people worldwide [7], slightly less than half of whom are women [8], and a high proportion of them already have or will have children [9]. Indeed, the incidence peak of the illness occurs twice in women aged 20–29 and 30–39 years [10] covering the childbearing period. In 1999, Denmark was the first country to publish population-based data on the outcomes of women with schizophrenia giving birth [11]. During the last two decades, Australia, Canada, Finland, France, Israel, Japan, Sweden, Taiwan, the UK, and the US have also published their national data, showing poor pregnancy and delivery outcomes for women with schizophrenia and increased neonatal complications and infant mortality for their newborns [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. However, a comprehensive meta‐analysis is lacking to produce robust and informative epidemiological estimates necessary to guide health policies to prioritise and organise perinatal services for women with schizophrenia.
Because new knowledge and empirical outcomes are needed to help perinatal care systems achieve high-quality care and decreased infant mortality and severe morbidity in women with schizophrenia [38], we carried out a systematic review and meta-analysis to synthesise the accumulating evidence on pregnancy, delivery, neonatal complications, and infant mortality among women with schizophrenia and their newborns.
Methods
Literature search strategy
This meta-analysis was conducted following the Preferred Reporting Items for Systematic reviews and Meta-Analysis guidelines [39]. Systematic bibliographic searches were carried out according to the Cochrane methodology. This project was registered in PROSPERO (reference number CRD42020197446).
The search paradigm was based on the Mesh terms of the Medline® database and adapted for Google Scholar® and Web of Science®: (‘schizophrenia’ or ‘psychotic disorders’) AND (‘pregnancy’ OR ‘delivery’ OR ‘obstetrical’ OR ‘neonatal’). The last search was carried out on September 30th, 2021. The reference lists of relevant reviews and articles were manually searched for additional eligible articles. If needed, the corresponding authors were asked to provide additional data that were not included in the original publications, and they were asked to provide any unpublished results.
Eligibility
The inclusion criteria were as follows: 1. Any language and date of publication; 2. Original research papers; 3. Population-based Studies 4. Case group: pregnant women diagnosed with schizophrenia-spectrum disorder based on an International Classification of Diseases (ICD) or Diagnostic and Statistical Manual (DSM) code. The schizophrenia-spectrum diagnosis was not necessarily reported before delivery. 5. Control group: women without schizophrenia-spectrum disorder or psychiatric disorder; 6. At least one quantitative pregnancy, delivery, neonatal or infant mortality outcome was reported in the case and control groups.
The exclusion criteria were: non-population-based studies (i.e. cohort or case–control studies based on a single department or hospital except if this hospital covered the whole geographical population area).
RM and DEE carried out the inclusion of studies. In the case of a non-consensus for the inclusion of a study, the last author (GF) made the final decision.
Pregnancy, delivery, neonatal, and infant mortality outcomes
The following pregnancy outcomes (15) were extracted: gestational diabetes; gestational hypertension; thromboembolism; urinary infection; anaemia; pre-eclampsia or eclampsia; placental abruption; antepartum haemorrhage; chorioamnionitis; threatened preterm labour; premature rupture of membrane; placental complication; oligohydramnios; polyhydramnios; and general infection during pregnancy.
The following delivery outcomes (7) were extracted: C-section; labour induction; instrumental delivery, postpartum haemorrhage; foetal distress; breech presentation, maternal death.
The following neonatal outcomes (11) were extracted: congenital malformations; asphyxia; Apgar score at 5 min <7; intensive care; small for gestational age (<10th percentile); large for gestational age (>90th percentile); low birth weight (<2500 g at birth or <10th percentile); high birth weight (>4000 g at birth or >90th percentile); preterm delivery (gestational age between 32 and 37 weeks); very preterm delivery (gestational age <32 weeks); and stillbirths.
The following infant mortality outcomes (3) were extracted: neonatal mortality (between 0 and 28 days of life); post-neonatal mortality (between 29 and 365 days of life); and infant death during the first year of life (0–365 days of life).
Other collected data
The following study characteristics were extracted: author; year of publication; year of first birth inclusion; year of last birth inclusion; country; database used; case definition (time for psychiatric diagnosis; diagnosis period covering the period before pregnancy; inclusion of post-delivery schizophrenia diagnoses; ICD or DSM codes); and control definition (women without schizophrenia; women without the psychiatric disorder).
Sociodemographic variables, comorbidities, treatments, and follow-up variables that could potentially affect pregnancy, delivery, neonatal, and child outcomes were also extracted: age; parity; absence of a partner in supportive environment (single; divorced or widowed); low socioeconomic level; ethnicity; primiparous pregnancy; child sex; smoking; alcohol; illicit drug; obesity; hypertension before pregnancy; diabetes before pregnancy; dysthyroid disorder; epilepsy; infection by human immunodeficiency virus; antipsychotic treatments, and the number of prenatal visits.
Three researchers (RM, DEE, and GF) extracted data from the included studies in a systematic manner using a predesigned extraction form, which was based on the Joanna Briggs Institute Data Extraction Form for Prevalence and Incidence Studies [40]. Additional items relevant to the current study were also added. The first and last authors (DEE and GF) examine each discrepancy in data extraction to reach consensus.
Quality assessment
Study quality analysis was performed using the NIH Study Quality Assessment Tools of case–control studies [41]. This tool was chosen to address the limits of the Newcastle-Ottawa scale [42].
Statistical analyses
Studies included in the present meta-analysis provided comparative data on pregnancy, delivery, neonatal and child outcomes for unmatched, and matched samples. Unmatched studies and matched studies were analysed separately. Using the inverse-variance weighting method, a random effects model was used to calculate the odds ratio (OR) of each outcome and its 95% confidence interval (95% CI) [43, 44]. When available, we used the numbers of events and the sample sizes instead of the OR [45]. Heterogeneity between studies was quantified with the I² statistic [46]. Q and I2 were calculated to assess heterogeneity across all studies and within subgroups, with I2 ≥ 50% indicating significant heterogeneity [47]. Publication bias was assessed graphically with a funnel plot and statistically with Egger’s test when at least ten studies were included in the meta-analysis [48]. Sensitivity analyses were conducted using the leave-one-out method [49].
Subgroup analyses for six binary variables (year of last inclusion < or ≥2009 (median year), high-quality studies or not, studies including only one birth per mother or not, studies including stillbirths or not, schizophrenia diagnosis before pregnancy vs. before and after pregnancy, and studies restricted to singleton births or not) were used to evaluate factors that moderated the individual study estimates of the OR of each outcome.
All analyses and graphs were carried out using R software with the meta and forest plot packages, respectively.
Results
Study and patient characteristics
Twenty-six studies from 11 high-income countries (43,611 deliveries of women with schizophrenia and 40,948,272 deliveries of control women) were included (Flow-chart Fig. 1, excluded studies Supplementary Table 1) [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. No study has been carried out in low- or middle-income countries. The following cohort and countries were included (in order of year of last patient included): Denmark (1973–1993), UK (1996–1998), Sweden (1983–2002), Taiwan (1999–2003), Israel (1998–2008), Japan (2005–2009), Canada ((Ontario)2002–2011), Finland (1991–2013), US (2002–2014, (California) 2005–2011), Australia (1980–1992, (Melbourne) 2008–2012, (Western Australia) 1980–2001, 2007–2017), and France (2015–2019). Their characteristics are presented in Table 1 and Supplementary Table 2 for unmatched studies (N = 21) and Table 2 and Supplementary Table 3 for matched studies (N = 6) (one study [12] was included in both unmatched and matched studies). Study quality is presented in Supplementary Table 4.
Unmatched studies
Twenty-one eligible studies, 38,680 deliveries of women with schizophrenia and 40,926,243 deliveries of control women, were included [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. Compared to controls, women with schizophrenia were found to be older (mean difference +1.74 years, 95% CI [0.47; 3.01], p = 0.007; I² = 96.2%, 95% CI [93.5–97.8]), more often without a partner (OR 3.78, 95% CI [3.08–4.65], p < 0.0001; I² = 84.6%, 95% CI [68.2–92.5]) and with a lower socioeconomic level (OR 1.77, 95% CI [1.30–2.42], p = 0.0003; I² = 98.8%, 95% CI [98.4–99.1]). They were more frequently smokers (OR 4.87, 95% CI [3.65–6.50], p < 0.0001; I² = 98%, 95% CI [97.3–98.5]) and illicit drug consumers (OR 13.33, 95% CI [8.65–20.54], p < 0.0001; I² = 93.2%, 95% CI [85.8–96.7]). They had more obesity (OR 2.38, 95% CI [1.78–3.19], p < 0.0001; I² = 84.4%, 95% CI [61.0–93.8]), hypertension before pregnancy (OR 1.77, 95% CI [1.51–2.07], p < 0.0001; I² = 11.2%, 95% CI [0.0–86.4]) and diabetes before pregnancy (OR 3.09, 95% CI [1.73–5.50], p < 0.0001; I² = 93.5%, 95% CI [86.5–96.8]). No significant difference was found for primiparous pregnancy (OR 1.07, 95% CI [0.94–1.21], p = 0.34; I² = 93.2%, 95% CI [89.3–95.7]) or child sex (OR 1.03, 95% CI [0.99–1.08], p = 0.16; I² = 0%, 95% CI [0.0–79.2]). Ethnicity, alcohol consumption, dysthyroid disorder, epilepsy, infection by human immunodeficiency virus, antipsychotic treatments and number of antenatal visits were not systematically collected and could not be analysed.
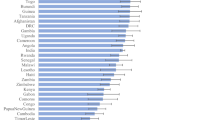
Of the 36 pregnancy, delivery, neonatal and child complications that were extracted, 20 were measured in 4 studies or more and were included in the quantitative analyses. The ORs for each outcome were compiled in a forest plot (Fig. 2).
Pregnancy outcomes
Compared to controls, women with schizophrenia had increased risk of gestational diabetes (OR 2.35, 95% CI [1.57–3.52], p < 0.001; I² = 80.4%, 95% CI [60.1–90.3]), gestational hypertension (OR 1.55, 95% CI [1.02–2.36], p = 0.041; I² = 85.5%, 95% CI [72.1–92.5]), pre-eclampsia or eclampsia (OR 1.85, 95% CI [1.52–2.25], p < 0.001; I² = 57.7%, 95% CI [2.0–81.7]), antepartum haemorrhage (OR 2.28, 95% CI [1.58–3.29], p < 0.001; I² = 54.9%, 95% CI [0.0–83.4]), placenta abruption (OR 2.20, 95% CI [2.02–2.39], p < 0.001; I² = 47.7%, 95% CI [0.0–77.9]), threatened preterm labour (OR 2.91, 95% CI [1.57–5.40], p < 0.001; I² = 88.8%, 95% CI [73.8–95.2]), and premature rupture of membrane (OR 1.29, 95% CI [1.06–1.58], p = 0.012; I² = 76.1%, 95% CI [46.4–89.4]).
Delivery outcomes
Compared to controls, women with schizophrenia had an increased risk of c-section (OR 1.33, 95% CI [1.22–1.45], p < 0.001; I² = 77.8%, 95% CI [56.1–88.7]), postpartum haemorrhage (OR 1.14, 95% CI [1.04–1.24], p = 0.003; I² = 0%, 95% CI [0.0–84.7]) and foetal distress (OR 1.80, 95% CI [1.43–2.26], p < 0.001; I² = 71.4%, 95% CI [87.5–96.1]). Women with schizophrenia had no statistically significant increased risk for breech presentation, labour induction or instrumental delivery (all p > 0.05).
Neonatal outcomes
Compared to controls, newborns of women with schizophrenia had increased risk of congenital malformations (OR 1.86, 95% CI [1.71–2.03], p < 0.001; I² = 39.2%, 95% CI [0.0–77.5]), Apgar score <7 at 5 min (OR 1.93, 95% CI [1.25–2.96], p = 0.003; I² = 56.7%, 95% CI [0.0–85.6]), small for gestational age (OR 1.63, 95% CI [1.48–1.80], p < 0.001; I² = 65%, 95% CI [28.5–82.8]), low birth weight (OR 1.75, 95% CI [1.46–2.11], p < 0.001; I² = 75.8%, 95% CI [53.5–87.4]), preterm delivery (OR 1.79, 95% CI [1.62–1.98], p < 0.001; I² = 76%, 95% CI [60.5–85.4]), very preterm delivery (OR 2.31, 95% CI [1.78–2.98], p < 0.001; I² = 47.6%, 95% CI [0.0–82.6]) and stillbirth (OR 2.06, 95% CI [1.83–2.31], p < 0.001; I² = 0%, 95% CI [0.0–67.6]). Stillbirth affected 0.96% of births (95% CI [0.74–1.25]; I² = 58.9%, 95% CI [10.3–81.2%]) in women with schizophrenia vs. 0.40% (95% CI [0.31–0.51]; I² = 99.9%) in controls. Women with schizophrenia had no statistically significant increased risk for large for gestational age and high birth weight (all p > 0.05).
Child outcomes
The following variables were significantly increased in children of women with schizophrenia compared to controls: neonatal death (OR 1.41, 95% CI [1.03–1.94], p = 0.033; I² = 0%, 95% CI [0.0–74.6]), post-neonatal death (OR 2.87, 95% CI [2.11–3.89], p < 0.001; I² = 0%, 95% CI [0.0–84.7]) and infant death (OR 2.33, 95% CI [1.81–3.01], p < 0.001; I² = 15.3%, 95% CI [0.0–82.4]). Infant death affected 1.05% of births (95% CI [0.83–1.33]; I² = 9.5%, 95% CI [0.0–81.2]) in women with schizophrenia vs. 0.44% of births (95% CI [0.38–0.51]; I² = 98.9%, 95% CI [98.4–99.2]) in controls.
Publication bias
Funnel plots and Egger’s test were performed only for preterm delivery measured in more than ten studies. This difference was not statistically significant (p > 0.05) (Supplementary Fig. 1).
Sensitivity analysis
Sensitivity analyses using the leave-one-out method found that these results were maintained after removing each of the included studies except for gestational hypertension, which became nonsignificant after removing the Frayne et al. study [31]. When omitting the study by Vigod et al. [18] the negative association between schizophrenia in mothers and high birth weight became significant (OR = 0.77 [0.60–0.99], p = 0.041; I² = 42.5%, 95% CI [0.0–80.7]).
Subgroup analyses (Table 2)
All results found in the global analyses were maintained in the subgroup analyses except for 6. First, the associations between tobacco smoking and preterm delivery with schizophrenia were stronger in studies with last inclusion in 2009 or after than in studies with last inclusions before 2009 (OR = 6.60 [4.90–8.89] vs. 3.61 [2.80–4.65], p = 0.003 and OR = 1.94 [1.74–2.16] vs. 1.62 [1.41–1.85], p = 0.041). In contrast, the association between low birth weight and schizophrenia was lower in studies with last inclusion in 2009 or after than in studies with last inclusions before 2009 (1.38 [1.20–1.59] vs. OR = 1.86 [1.52–2.28], p = 0.018). No significant difference between associations between schizophrenia and the following outcomes was found between studies with the year of last inclusion in 2009 or after vs. before 2009: low socioeconomic level, primiparous pregnancy, gestational diabetes, placental abruption, small for gestational age, and stillbirth. The other outcomes could not be analysed due to a lack of data.
Second, the association between schizophrenia in the mother and foetal distress was higher in studies including single pregnancy per mother than those including multiple pregnancies (OR = 3.55 [2.50–5.03] vs. OR = 1.35 [1.30–1.40], p < 0.001). A negative association between schizophrenia in mothers and large for gestational age was found in studies including single pregnancy per mother but not in those including multiple pregnancies (OR = 0.63 [0.56–0.71] vs. OR = 1.38 [0.93–2.05], p = <0.001).
Third, another negative association between schizophrenia in mother and instrumental delivery was found in studies including stillbirths but not in those excluding stillbirths (OR = 0.73 [0.62–0.86] vs. 1.21 [0.77–1.89], p = 0.040) and in high-quality studies but not in low- to moderate-quality studies (OR = 0.73 [0.62–0.86] vs. 1.21 [0.77–1.89], p = 0.040).
Matched studies
Six matched studies were included (7590 deliveries of women with schizophrenia and 32,272 deliveries of control women) [12, 33,34,35,36,37]. Five studies were matched for age [12, 34,35,36,37], and 3 were matched for age and geographical location [12, 36, 37]. The following associations found in unmatched studies were replicated in matched studies: schizophrenia in the mother was associated with increased gestational hypertension (OR 1.32, 95% CI [1.07–1.61], I² = 0%, 95% CI [0.0–79.2]) and congenital malformations (OR 1.33, 95% CI [1.15–1.53], I² = 0%, 95% CI [0.0–79.2]). The other variables could not be analysed due to a lack of data.
Publication bias
Funnel plots and Egger’s tests were not performed because the number of studies was <10.
Sensitivity analysis
Sensitivity analyses using the leave-one-out method found that these results were maintained after removing each of the included studies except for the association between schizophrenia and gestational hypertension, which became nonsignificant (p > 0.05) after removing Fabre et al. [12] matched study (Table 3).
Subgroup analysis
Due to the small number of matched studies, no subgroup analyses were performed.
Discussion
This large-scale meta-analysis is the first to synthesise the accumulating evidence on pregnancy, delivery, neonatal complications, and infant mortality among women with schizophrenia and their newborns. A recent review highlighted the opportunity for real-world data from medico-administrative and electronic medical records databases to obtain for the first time a large sample of pregnant women with schizophrenia, longitudinal follow-up, and findings on rare outcomes [50]. Our meta-analysis included data from over 40 million people in 11 high-income countries published during the last two decades. Given the importance of health disparities between women with and without schizophrenia, further investment, and research into preventing preterm delivery, stillbirth, and infant mortality are needed.
These health disparities have appeared relatively stable since 1999 despite important investments in maternal and infant health care systems. In Australia, a universal psychosocial assessment was developed in maternal and infant health care systems in the 2000s [51]. The enactment of the Affordable Care Act in 2010 has been associated with great improvements in women’s perinatal mental health care in the US [52]. Since 2016, the UK has invested >£400 million into new specialist perinatal mental health to ensure that women in all parts of the UK have access to specialist community services and psychiatric inpatient mother and baby units and extending service provision up to 2 years postpartum [53,54,55,56]. In France, three perinatal plans were implemented in 1970, 1995, and 2005. These programmes have targeted care access for all women with a special focus on depression and postpartum psychosis but nothing dedicated to women with schizophrenia. Our findings suggest that these programmes are insufficient to address the complex needs of women with schizophrenia and that programmes based on tailor-made and intensive care could be the key.
Integrated interventions appear essential for holistic perinatal care, but relatively few have been developed and assessed thus far [5]. To reduce care fragmentation, these integrated interventions should include multiple professionals with a case manager at the same unit of time and place in a patient-centred approach. At the same time, women with schizophrenia are currently referred to separate services [12]. In 2019, a francophone alliance for perinatal health was created to support the development of primary care in perinatal mental health integrated into a graduated care system, implementing systematised psychosocial assessments, building a system that integrates all health disciplines and primary, secondary and tertiary perinatal care, and providing continuous, coordinated and graduated interventions (from preconception to postnatal) [57].
There is evidence that a high proportion of pregnancies are unplanned for schizophrenia, promoting pre-conceptional interventions within usual care for all women with schizophrenia [58]. A preconception conceptual framework may include a biological perspective (the days to weeks before embryo development), an individual perspective (a conscious intention to conceive) and a public health perspective (months or years beforehand) to address preconception risk factors such as diet and obesity [58]. Although not all women want to be mothers, the pregnancy perspective may be a strong motivational factor to prevent risky health behaviours and metabolic comorbidities in some patients [5]. We have identified addictive behaviours (i.e. tobacco smoking and illicit drug consumption) and metabolic comorbidities (i.e. obesity, hypertension, and diabetes) as major issues in women with schizophrenia. The association between tobacco smoking and schizophrenia was stronger in studies with the last year of inclusion in 2009 or after vs. those before 2009, suggesting that women with schizophrenia may not have benefitted from public health interventions or other interventions for tobacco smoking than other women without schizophrenia. Optimising healthy lifestyle interventions is needed, as the Swedish MINT and the UK INTERaCT and STEPWISE interventions delivered to patients with schizophrenia from both sexes reported disappointing results [59, 60]. In addition to reduced care fragmentation, including fathers [61] and family caregivers [62], may improve the effectiveness of these programmes. Other effective interventions (i.e. nicotine substitute and varenicline [63], N-acetylcysteine [64], omega 3 fatty acids [65], metformin and/or topiramate [66], statins, fenofibrate, and adipose tissue surgical reduction [67]) are insufficiently provided in usual care for multiple reasons [68]. While probably frequent, we lack data on alcohol consumption in women with schizophrenia [69]. Alcohol consumption is often associated with other addictions [70] and should also be addressed.
Mother and baby units have improved the clinical and social outcomes of women. However, women with schizophrenia with low social support and low socioeconomic level have lower chances of having improved outcomes after discharge from these units [71]. We confirmed that women with schizophrenia were more frequently socially deprived and without partners than those without schizophrenia. Clinical studies have shown more frequent exposure to domestic violence, childhood adversity, and other stress factors [72,73,74,75,76,77,78]. Psychosocial interventions are recommended in adjunction to pharmacological treatments and psychotherapies [79] and could be individual or in groups, including peer support or peer support groups [80, 81]. The development of video sessions has been considerably accelerated with the COVID-19 pandemic and maybe an opportunity to improve coordination and care access [82].
Limitations and perspectives. Our results show that women with schizophrenia have an increased risk of very preterm delivery. We do not know if these very preterm deliveries are spontaneous or induced. The increased c-section rate in schizophrenia is in favour of induced preterm delivery. Increased urogenital infections in women with schizophrenia may also increase spontaneous preterm delivery and could be prevented [12, 36]. Additional data on the causes and interventions to prevent preterm deliveries are needed. Early and frequent antenatal visits are recommended to adapt treatments and prevent pregnancy complications, but we lack data on the care access of women with schizophrenia [12]. We also lack data on antipsychotic exposure, which may increase the risk of gestational diabetes in mothers [83, 84] and congenital malformation in newborns [85,86,87,88]. They probably have no role in low birth weight, small for gestational age [89] or stillbirth [90]. We lack data on trimester exposure to antipsychotics, that may also modulate the risk for the new born. Trimesters are rarely reported in the population-based studies as the conception date is missing in the national databases. These risks should be balanced with uncontrolled/untreated schizophrenia that may increase all risky health behaviours, delayed antenatal follow-up and congenital malformations [12, 91]. Some countries have published only matched or unmatched analyses. Matched data are difficult to include in meta-analyses due to multiple matching factors varying across studies. It seems recommended for future studies to publish both matched and unmatched data, as these analyses yield complementary evidence. Most of national databases have not been designed for clinical studies and use the ICD codes for diagnoses. Tobacco smoking is probably underreported as it is often a secondary diagnosis and as the F17* codes focus on tobacco consumption adverse events (craving, withdrawal symptoms and addictive behaviour). However, all smokers could be considered as having a problematic tobacco use. National databases should address this issue to improve the coding of tobacco consumption and better identify tobacco smokers. However, we may hypothesise that tobacco smoking may be better coded in pregnant women, as tobacco smoking increase the risk of pregnancy, delivery and neonatal complications. The analysis of administrative databases must be complemented by qualitative approaches to identify patients’ needs and expectations. Pregnancy denial is a rare and serious event that is also unreported and should be further explored [92]. Unwanted pregnancy is more frequent and may also increase poor pregnancy’s outcomes [93]. Our results have raised complex ethical questions for the prevention of poor health outcomes in both mothers with schizophrenia and their children. These ethical issues would benefit from an expert consensus to guide clinical practice. It is necessary to have analyses of the pathways/trajectories of patients before/during/after pregnancy. Longitudinal analyses must now complement these cross-sectional approaches. Network research should be encouraged due to the small number of cases per site. International studies should be carried out on this subject.
Conclusion
This large‐scale meta‐analysis confirms that schizophrenia is associated with a substantially increased risk of very preterm delivery, stillbirth, and infant mortality, and metabolic risk in mothers. Integrated patient-centred care interventions are needed to address the complex social and biological needs of women with schizophrenia and their newborns, and define best practices. On a global health agenda, we lack data on low- and middle-income countries. At the same time, other studies converge to show that the health problems of women with schizophrenia are even more serious in these countries than in high-income countries [5]. Women with schizophrenia should be listed as a priority in the mental health agenda.
References
Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, et al. Effects of perinatal mental disorders on the fetus and child. Lancet Lond Engl. 2014;384:1800–19.
Howard LM, Molyneaux E, Dennis C-L, Rochat T, Stein A, Milgrom J. Non-psychotic mental disorders in the perinatal period. Lancet Lond Engl. 2014;384:1775–88.
Jones I, Chandra PS, Dazzan P, Howard LM. Bipolar disorder, affective psychosis, and schizophrenia in pregnancy and the post-partum period. Lancet. 2014;384:1789–99.
WHO. Maternal mental health. 2021. https://www.who.int. Accessed 18 Dec 2021.
Howard LM, Khalifeh H. Perinatal mental health: a review of progress and challenges. World Psychiatry. 2020;19:313–27.
Campo JV, Fontanella CA, Bridge JA. Intergenerational associations of parental mental illness and child health. JAMA Pediatr. 2020;174:e201755.
Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–22.
Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2:e141.
Howard LM, Kumar R, Thornicroft G. Psychosocial characteristics and needs of mothers with psychotic disorders. Br J Psychiatry J Ment Sci. 2001;178:427–32.
van der Werf M, Hanssen M, Köhler S, Verkaaik M, Verhey FR, RISE Investigators. et al. Systematic review and collaborative recalculation of 133,693 incident cases of schizophrenia. Psychol Med. 2014;44:9–16.
Bennedsen BE, Mortensen PB, Olesen AV, Henriksen TB. Preterm birth and intra-uterine growth retardation among children of women with schizophrenia. Br J Psychiatry J Ment Sci. 1999;175:239–45.
Fabre C, Pauly V, Baumstarck K, Etchecopar-Etchart D, Orleans V, Llorca P-M, et al. Pregnancy, delivery and neonatal complications in women with schizophrenia: a national population-based cohort study. Lancet Reg Health Eur. 2021;10:100209.
Ellman LM, Huttunen M, Lönnqvist J, Cannon TD. The effects of genetic liability for schizophrenia and maternal smoking during pregnancy on obstetric complications. Schizophr Res. 2007;93:229–36.
Nilsson E, Lichtenstein P, Cnattingius S, Murray R, Hultman C. Women with schizophrenia: pregnancy outcome and infant death among their offspring. Schizophr Res. 2002;58:221–9.
Nilsson E, Hultman CM, Cnattingius S, Olausson PO, Björk C, Lichtenstein P. Schizophrenia and offspring’s risk for adverse pregnancy outcomes and infant death. Br J Psychiatry. 2008;193:311–5.
Männistö T, Mendola P, Kiely M, O’Loughlin J, Werder E, Chen Z, et al. Maternal psychiatric disorders and risk of preterm birth. Ann Epidemiol. 2016;26:14–20.
Baer RJ, Chambers CD, Bandoli G, Jelliffe-Pawlowski LL. Risk of preterm birth by subtype among Medi-Cal participants with mental illness. Am J Obstet Gynecol. 2016;215:519.e1–9.
Vigod S, Kurdyak P, Dennis C, Gruneir A, Newman A, Seeman M, et al. Maternal and newborn outcomes among women with schizophrenia: a retrospective population-based cohort study. BJOG Int J Obstet Gynaecol. 2014;121:566–74.
Vigod SN, Fung K, Amartey A, Bartsch E, Felemban R, Saunders N, et al. Maternal schizophrenia and adverse birth outcomes: what mediates the risk? Soc Psychiatry Psychiatr Epidemiol. 2020;55:561–70.
Zhong Q-Y, Gelaye B, Fricchione GL, Avillach P, Karlson EW, Williams MA. Adverse obstetric and neonatal outcomes complicated by psychosis among pregnant women in the United States. BMC Pregnancy Childbirth. 2018;18:120.
Heun-Johnson H, Seabury SA, Menchine M, Claudius I, Axeen S, Lakshmanan A. Association between maternal serious mental illness and adverse birth outcomes. J Perinatol. 2019;39:737–45.
Jablensky AV, Morgan V, Zubrick SR, Bower C, Yellachich L-A. Pregnancy, delivery, and neonatal complications in a population cohort of women with schizophrenia and major affective disorders. Am J Psychiatry. 2005;162:79–91.
Bennedsen BE, Mortensen PB, Olesen AV, Henriksen TB. Congenital malformations, stillbirths, and infant deaths among children of women with schizophrenia. Arch Gen Psychiatry. 2001;58:674.
Di Prinzio P, Morgan VA, Björk J, Croft M, Lin A, Jablensky A, et al. Intellectual disability and psychotic disorders in children: association with maternal severe mental illness and exposure to obstetric complications in a whole-population cohort. Am J Psychiatry. 2018;175:1232–42.
Liu T-C, Chen C-S, Loh CPA. Do children of parents with mental illness have lower survival rate? A population-based study. Compr Psychiatry. 2010;51:250–5.
Lee H-C, Lin H-C. Maternal bipolar disorder increased low birthweight and preterm births: a nationwide population-based study. J Affect Disord. 2010;121:100–5.
Hizkiyahu R, Levy A, Sheiner E. Pregnancy outcome of patients with schizophrenia. Am J Perinatol. 2010;27:19–23.
Nguyen NT, Gorman M, Caughey AB. Pregnancy outcomes in women with schizophrenia: a retrospective cohort study. Am J Obstet Gynecol. 2016;214:S296.
Hironaka M, Kotani T, Sumigama S, Tsuda H, Mano Y, Hayakawa H, et al. Maternal mental disorders and pregnancy outcomes: a clinical study in a Japanese population: perinatal outcomes of mental disorders. J Obstet Gynaecol Res. 2011;37:1283–9.
Nguyen TN, Faulkner D, Frayne JS, Allen S, Hauck YL, Rock D, et al. Obstetric and neonatal outcomes of pregnant women with severe mental illness at a specialist antenatal clinic. Med J Aust. 2013;199:S26–9.
Frayne J, Nguyen T, Allen S, Hauck Y, Liira H, Vickery A. Obstetric outcomes for women with severe mental illness: 10 years of experience in a tertiary multidisciplinary antenatal clinic. Arch Gynecol Obstet. 2019;300:889–96.
Judd F, Komiti A, Sheehan P, Newman L, Castle D, Everall I. Adverse obstetric and neonatal outcomes in women with severe mental illness: To what extent can they be prevented? Schizophr Res. 2014;157:305–9.
Suvisaari JM, Taxell-Lassas V, Pankakoski M, Haukka JK, Lönnqvist JK, Häkkinen LT. Obstetric complications as risk factors for schizophrenia spectrum psychoses in offspring of mothers with psychotic disorder. Schizophr Bull. 2013;39:1056–66.
Howard LM, Goss C, Leese M, Thornicroft G. Medical outcome of pregnancy in women with psychotic disorders and their infants in the first year after birth. Br J Psychiatry. 2003;182:63–7.
Lin H-C, Chen Y-H, Lee H-C. Prenatal Care and adverse pregnancy outcomes among women with schizophrenia: a nationwide population-based study in Taiwan. J Clin Psychiatry. 2009;70:1297–303.
Simoila L, Isometsä E, Suvisaari J, Halmesmäki E, Lindberg N. Obstetric and perinatal health outcomes related to schizophrenia: A national register-based follow-up study among Finnish women born between 1965 and 1980 and their offspring. Eur Psychiatry. 2018;52:68–75.
Simoila L, Isometsä E, Gissler M, Suvisaari J, Sailas E, Halmesmäki E, et al. Maternal schizophrenia and out-of-home placements of offspring: a national follow-up study among Finnish women born 1965–1980 and their children. Psychiatry Res. 2019;273:9–14.
Barfield WD. Improving systems in perinatal care: quality, not quantity. JAMA. 2012;307:1750–1.
PRISMA-P Group, Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1.
Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Health. 2015;13:147–53.
National Institutes of Health (NIH). Study Quality Assessment Tools. https://www.nhlbi.nih.gov/healthtopics/study-quality-assessment-tools. Accessed 05-07-2022.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5.
Hartung J, Knapp G, Sinha BK. Statistical meta-analysis with applications. Hoboken, N.J: Wiley; 2008.
Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58.
Chang B-H, Hoaglin DC. Meta-analysis of odds ratios: current good practices. Med Care. 2017;55:328–35.
Higgins JPT. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Bedford J, Farrar J, Ihekweazu C, Kang G, Koopmans M, Nkengasong J. A new twenty-first century science for effective epidemic response. Nature. 2019;575:130–6.
Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34.
Cooper HM, Hedges LV, Valentine JC, editors. The handbook of research synthesis and meta-analysis. 2nd ed. New York: Russell Sage Foundation; 2009.
Taylor CL, Munk-Olsen T, Howard LM, Vigod SN. Schizophrenia around the time of pregnancy: leveraging population-based health data and electronic health record data to fill knowledge gaps. BJPsych Open. 2020;6:e97.
Austin M-PV, Middleton PF, Highet NJ. Australian mental health reform for perinatal care. Med J Aust. 2011;195:112–3.
Lee LK, Chien A, Stewart A, Truschel L, Hoffmann J, Portillo E, et al. Women’s coverage, utilization, affordability, and health after the ACA: a review of the literature. Health Aff Proj Hope. 2020;39:387–94.
The Perinatal Mental Health Care Pathways :Full implementation guidance. https://www.rcpsych.ac.uk/docs/default-source/improvingcare/nccmh/perinatal/nccmh-the-perinatal-mental-health-care-pathways-full-implementation-guidance.pdf?sfvrsn=73c19277_2. Accessed 05-07-2022.
Centre of Perinatal Excellence (COPE) - Mental Health Care in the Perinatal Period :Australian clinical Practice Guideline. https://cope.org.au/wpcontent/uploads/2017/10/Final-COPE-Perinatal-Mental-Health-Guideline.pdf. Accessed 05-07-2022
National Collaborating Centre for Mental Health (UK). Antenatal and Postnatal Mental Health: The NICE Guideline on Clinical Management and Service Guidance. Leicester (UK): British Psychological Society; 2007.
GOV.UK. Welcome to GOV.UK. 2021. https://www.gov.uk/. Accessed 27 April 2021.
Une alliance francophone pour la santé mentale périnatale. Gynger. https://www.gynger.fr/une-alliance-francophone-pour-la-sante-mentale-perinatale/. Accessed 18 December 2021.
Catalao R, Mann S, Wilson C, Howard LM. Preconception care in mental health services: planning for a better future. Br J Psychiatry J Ment Sci. 2020;216:180–1.
Gossage-Worrall R, Hind D, Barnard-Kelly KD, Shiers D, Etherington A, Swaby L, et al. STructured lifestyle education for people WIth SchizophrEnia (STEPWISE): mixed methods process evaluation of a group-based lifestyle education programme to support weight loss in people with schizophrenia. BMC Psychiatry. 2019;19:358.
Lovell K, Wearden A, Bradshaw T, Tomenson B, Pedley R, Davies LM, et al. An exploratory randomized controlled study of a healthy living intervention in early intervention services for psychosis: the INTERvention to encourage ACTivity, improve diet, and reduce weight gain (INTERACT) study. J Clin Psychiatry. 2014;75:498–505.
Seedat S. Paternal perinatal mental disorders are inextricably linked to maternal and child morbidity. World Psychiatry. 2020;19:337–8.
Sledge WH, Astrachan B, Thompson K, Rakfeldt J, Leaf P. Case management in psychiatry: an analysis of tasks. Am J Psychiatry. 1995;152:1259–65.
Siskind DJ, Wu BT, Wong TT, Firth J, Kisely S. Pharmacological interventions for smoking cessation among people with schizophrenia spectrum disorders: a systematic review, meta-analysis, and network meta-analysis. Lancet Psychiatry. 2020;7:762–74.
Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia–a double-blind, randomized, placebo-controlled trial. Biol Psychiatry. 2008;64:361–8.
Tang W, Wang Y, Xu F, Fan W, Zhang Y, Fan K, et al. Omega-3 fatty acids ameliorate cognitive dysfunction in schizophrenia patients with metabolic syndrome. Brain Behav Immun. 2020;88:529–34.
Zheng W, Zhang QE, Cai DB, Yang XH, Ungvari GS, Ng CH, et al. Combination of metformin and lifestyle intervention for antipsychotic-related weight gain: a meta-analysis of randomized controlled trials. Pharmacopsychiatry. 2019;52:24–31.
Sperling LS, Mechanick JI, Neeland IJ, Herrick CJ, Després J-P, Ndumele CE, et al. The cardiometabolic health alliance: working toward a new care model for the metabolic syndrome. J Am Coll Cardiol. 2015;66:1050–67.
Marynak K, VanFrank B, Tetlow S, Mahoney M, Phillips E, Jamal Mbbs A, et al. Tobacco cessation interventions and smoke-free policies in mental health and substance abuse treatment facilities—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67:519–23.
Archibald L, Brunette MF, Wallin DJ, Green AI. Alcohol Use Disorder and Schizophrenia or Schizoaffective Disorder. Alcohol Res Curr Rev. 2019;40:arcr.v40.1.06.
Tsang TW, Elliott EJ. High global prevalence of alcohol use during pregnancy and fetal alcohol syndrome indicates need for urgent action. Lancet Glob Health. 2017;5:e232–3.
Glangeaud-Freudenthal NM-C. Mother-Baby psychiatric units (MBUs): national data collection in France and in Belgium (1999-2000). Arch Womens Ment Health. 2004;7:59–64.
Dean K, Walsh E, Moran P, Tyrer P, Creed F, Byford S, et al. Violence in women with psychosis in the community: prospective study. Br J Psychiatry J Ment Sci. 2006;188:264–70.
Wan MW, Salmon MP, Riordan DM, Appleby L, Webb R, Abel KM. What predicts poor mother-infant interaction in schizophrenia? Psychol Med. 2007;37:537–46.
Rice E. The invisibility of violence against women diagnosed with schizophrenia: a synthesis of perspectives. ANS Adv Nurs Sci. 2008;31:E9–21.
Kelly DL, Rowland LM, Patchan KM, Sullivan K, Earl A, Raley H, et al. Schizophrenia clinical symptom differences in women vs. men with and without a history of childhood physical abuse. Child Adolesc Psychiatry Ment Health. 2016;10:5.
Afe TO, Emedoh TC, Ogunsemi OO, Adegohun AA. Socio-demographic characteristics, partner characteristics, socioeconomic variables, and intimate partner violence in women with schizophrenia in south-south Nigeria. J Health Care Poor Underserved. 2017;28:707–20.
Hui CLM, Ko WT, Chang WC, Lee EHM, Chan SKW, Chen EYH. Clinical and functional correlates of financially deprived women with first-episode psychosis. Early Inter Psychiatry. 2019;13:639–45.
Prokopez CR, Cesoni OM, Caporusso GB, Reffino-Pereyra ML, Alberio G, Vallejos M Prevalence and clinical impact of childhood adversities in women with schizophrenia. Clin Schizophr Relat Psychoses. 2018. https://doi.org/10.3371/CSRP.PRCE.061518.
Valencia M, Fresan A, Juárez F, Escamilla R, Saracco R. The beneficial effects of combining pharmacological and psychosocial treatment on remission and functional outcome in outpatients with schizophrenia. J Psychiatr Res. 2013;47:1886–92.
Chien WT, Clifton AV, Zhao S, Lui S. Peer support for people with schizophrenia or other serious mental illness. Cochrane Database Syst Rev. 2019;4:CD010880.
Castelein S, Bruggeman R, Davidson L, van der Gaag M. Creating a supportive environment: peer support groups for psychotic disorders. Schizophr Bull. 2015;41:1211–3.
Soukup T, Sevdalis N, Green JSA, Lamb BW. Quality improvement for cancer multidisciplinary teams: lessons learned from the Anglian Germ Cell Cancer Collaborative Group. Br J Cancer. 2021;124:313–4.
Wang Z, Wong ICK, Man KKC, Alfageh BH, Mongkhon P, Brauer R. The use of antipsychotic agents during pregnancy and the risk of gestational diabetes mellitus: a systematic review and meta-analysis. Psychol Med. 2021;51:1028–37.
Gentile S. Antipsychotic therapy during early and late pregnancy. A systematic review. Schizophr Bull. 2010;36:518–44.
Terrana N, Koren G, Pivovarov J, Etwel F, Nulman I. Pregnancy outcomes following in utero exposure to second-generation antipsychotics: a systematic review and meta-Analysis. J Clin Psychopharmacol. 2015;35:559–65.
Ennis ZN, Damkier P. Pregnancy exposure to olanzapine, quetiapine, risperidone, aripiprazole and risk of congenital malformations. A systematic review. Basic Clin Pharmacol Toxicol. 2015;116:315–20.
Andrade C. Major congenital malformations associated with exposure to second-generation antipsychotic drugs during pregnancy. J Clin Psychiatry. 2021;82:21f14252.
Fond G, Etchecopar-Etchart D, Blanc J, Boyer L. Antipsychotics during pregnancy and increased risk of congenital malformation in offspring: toward a systematic use of real-world data. Lancet Reg Health Eur. 2021;11:100257.
Lin H-C, Chen I-J, Chen Y-H, Lee H-C, Wu F-J. Maternal schizophrenia and pregnancy outcome: does the use of antipsychotics make a difference? Schizophr Res. 2010;116:55–60.
Coughlin CG, Blackwell KA, Bartley C, Hay M, Yonkers KA, Bloch MH. Obstetric and neonatal outcomes after antipsychotic medication exposure in pregnancy. Obstet Gynecol. 2015;125:1224–35.
Tosato S, Albert U, Tomassi S, Iasevoli F, Carmassi C, Ferrari S, et al. A systematized review of atypical antipsychotics in pregnant women: balancing between risks of untreated illness and risks of drug-related adverse effects. J Clin Psychiatry. 2017;78:e477–89.
Jenkins A, Millar S, Robins J. Denial of pregnancy: a literature review and discussion of ethical and legal issues. J R Soc Med. 2011;104:286–91.
McNeil TF, Schubert EW, Cantor-Graae E, Brossner M, Schubert P, Henriksson KM. Unwanted pregnancy as a risk factor for offspring schizophrenia-spectrum and affective disorders in adulthood: a prospective high-risk study. Psychol Med. 2009;39:957–65.
Acknowledgements
DUBA Audrey, Department of Child Psychiatry, Assistance Publique—Hôpitaux Marseille, Marseille, France. BLANC Julie, Department of Obstetrics and Gynaecology, APHM, Nord Hospital, Marseille, France. This work was funded by the FondaMental Foundation, Assistance Publique—Hôpitaux Marseille (APHM) and Aix-Marseille University (AMU).
Author information
Authors and Affiliations
Contributions
Concept and design: LB, GF. Acquisition and analysis: DEE, RM, GF, LB. Interpretation of data: DEE, RM, JB, LB, GF. Drafting of the paper: DEE, LB, GF. Critical revision of the paper for important intellectual content: All the authors. Statistical analysis: DEE, LB. Supervision: LB.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Etchecopar-Etchart, D., Mignon, R., Boyer, L. et al. Schizophrenia pregnancies should be given greater health priority in the global health agenda: results from a large-scale meta-analysis of 43,611 deliveries of women with schizophrenia and 40,948,272 controls. Mol Psychiatry 27, 3294–3305 (2022). https://doi.org/10.1038/s41380-022-01593-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01593-9