Abstract
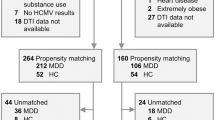
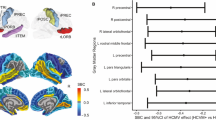
Human cytomegalovirus (HCMV) infection is associated with neuropathology in patients with impaired immunity and/or inflammatory diseases. However, the association between gray matter volume (GMV) and HCMV has never been examined in major depressive disorder (MDD) despite the presence of inflammation and impaired viral immunity in a subset of patients. We tested this relationship in two independent samples consisting of 179 individuals with MDD and 41 healthy controls (HC) (sample 1) and 124 MDD participants and 148 HCs (sample 2). HCMV positive (HCMV+) and HCMV negative (HCMV-) groups within each sample were balanced on up to 11 different clinical/demographic variables using inverse probability of treatment weighting. GMV of 87 regions was measured with FreeSurfer. There was a main effect of HCMV serostatus but not diagnosis that replicated across samples. Relative to HCMV- subjects, HCMV+ subjects in sample 1 showed a significant reduction of volume in six regions (puncorrected < 0.05). The reductions in GMV of the right supramarginal gyrus (standardized beta coefficient (SBC) = −0.26) and left fusiform gyrus (SBC = −0.25) in sample 1 were replicated in sample 2: right supramarginal gyrus (puncorrected < 0.05, SBC = −0.32), left fusiform gyrus (PFDR < 0.01, SBC = −0.51). Posthoc tests revealed that the effect of HCMV was driven by differences between the HCMV+ and HCMV- MDD subgroups. HCMV IgG level, a surrogate marker of viral activity, was correlated with GMV in the left fusiform gyrus (r = −0.19, Puncorrected = 0.049) and right supramarginal gyrus (r = −0.19, puncorrected = 0.043) in the HCMV+ group of sample 1. Conceivably, HCMV infection may be a treatable source of neuropathology in vulnerable MDD patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The full analysis code, datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Tsutsui Y, Kosugi I, Kawasaki H. Neuropathogenesis in cytomegalovirus infection: indication of the mechanisms using mouse models. Rev Med Virol. 2005;15:327–45.
Poland SD, Costello P, Dekaban GA, Rice GP. Cytomegalovirus in the brain: in vitro infection of human brain-derived cells. J Infect Dis. 1990;162:1252–62.
Alcendor DJ, Charest AM, Zhu WQ, Vigil HE, Knobel SM. Infection and upregulation of proinflammatory cytokines in human brain vascular pericytes by human cytomegalovirus. J Neuroinflammation. 2012;9:95.
Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev. 2013;26:86–102.
Pariante CM, Carpiniello B, Orru MG, Sitzia R, Piras A, Farci AM, et al. Chronic caregiving stress alters peripheral blood immune parameters: the role of age and severity of stress. Psychother Psychosom. 1997;66:199–207.
Glaser R, Kiecolt-Glaser JK, Speicher CE, Holliday JE. Stress, loneliness, and changes in herpesvirus latency. J Behav Med. 1985;8:249–60.
Docke WD, Prosch S, Fietze E, Kimel V, Zuckermann H, Klug C, et al. Cytomegalovirus reactivation and tumour necrosis factor. Lancet. 1994;343:268–9.
Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. Jama. 2008;300:413–22.
Iglesias-Escudero M, Moro-Garcia MA, Marcos-Fernandez R, Garcia-Torre A, Alvarez-Arguelles ME, Suarez-Fernandez ML, et al. Levels of anti-CMV antibodies are modulated by the frequency and intensity of virus reactivations in kidney transplant patients. PLoS ONE. 2018;13:e0194789.
Prosch S, Wendt CE, Reinke P, Priemer C, Oppert M, Kruger DH, et al. A novel link between stress and human cytomegalovirus (HCMV) infection: sympathetic hyperactivity stimulates HCMV activation. Virology. 2000;272:357–65.
Sarid O, Anson O, Yaari A, Margalith M. Academic stress, immunological reaction, and academic performance among students of nursing and physiotherapy. Res Nurs Health. 2004;27:370–7.
Mehta SK, Stowe RP, Feiveson AH, Tyring SK, Pierson DL. Reactivation and shedding of cytomegalovirus in astronauts during spaceflight. J Infect Dis. 2000;182:1761–4.
Mehta SK, Laudenslager ML, Stowe RP, Crucian BE, Sams CF, Pierson DL. Multiple latent viruses reactivate in astronauts during Space Shuttle missions. Brain Behav Immun. 2014;41:210–7.
Janicki-Deverts D, Cohen S, Doyle WJ, Marsland AL, Bosch J. Childhood environments and cytomegalovirus serostatus and reactivation in adults. Brain Behav Immun. 2014;40:174–81.
Fagundes CP, Glaser R, Malarkey WB, Kiecolt-Glaser JK. Childhood adversity and herpesvirus latency in breast cancer survivors. Health Psychol. 2013;32:337–44.
Shirtcliff EA, Coe CL, Pollak SD. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. Proc Natl Acad Sci USA. 2009;106:2963–7.
Rooney BV, Crucian BE, Pierson DL, Laudenslager ML, Mehta SK. Herpes Virus Reactivation in Astronauts During Spaceflight and Its Application on Earth. Front Microbiol. 2019;10:16.
Bano A, Pera A, Almoukayed A, Clarke THS, Kirmani S, Davies KA et al. CD28 (null) CD4 T-cell expansions in autoimmune disease suggest a link with cytomegalovirus infection. F1000Res. 2019;8:F1000 Faculty Rev-327.
Hunt PW, Martin JN, Sinclair E, Epling L, Teague J, Jacobson MA, et al. Valganciclovir reduces T cell activation in HIV-infected individuals with incomplete CD4+ T cell recovery on antiretroviral therapy. J Infect Dis. 2011;203:1474–83.
Zivadinov R, Chin J, Horakova D, Bergsland N, Weinstock-Guttman B, Tamano-Blanco M, et al. Humoral responses to herpesviruses are associated with neurodegeneration after a demyelinating event: results from the multi-center set study. J Neuroimmunol. 2014;273:58–64.
Lurain NS, Hanson BA, Martinson J, Leurgans SE, Landay AL, Bennett DA, et al. Virological and immunological characteristics of human cytomegalovirus infection associated with Alzheimer disease. J Infect Dis. 2013;208:564–72.
Dantzer R. Neuroimmune Interactions: From the Brain to the Immune System and Vice Versa. Physiol Rev. 2018;98:477–504.
Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34.
Mechawar N, Savitz J. Neuropathology of mood disorders: do we see the stigmata of inflammation? Transl Psychiatry. 2016;6:e946.
Appels A, Bar FW, Bar J, Bruggeman C, de Baets M. Inflammation, depressive symptomtology, and coronary artery disease. Psychosom Med. 2000;62:601–5.
Rector JL, Dowd JB, Loerbroks A, Burns VE, Moss PA, Jarczok MN, et al. Consistent associations between measures of psychological stress and CMV antibody levels in a large occupational sample. Brain Behav Immun. 2014;38:133–41.
Miller GE, Freedland KE, Duntley S, Carney RM. Relation of depressive symptoms to C-reactive protein and pathogen burden (cytomegalovirus, herpes simplex virus, Epstein-Barr virus) in patients with earlier acute coronary syndromes. Am J Cardiol. 2005;95:317–21.
Dickerson F, Wilcox HC, Adamos M, Katsafanas E, Khushalani S, Origoni A, et al. Suicide attempts and markers of immune response in individuals with serious mental illness. J Psychiatr Res. 2017;87:37–43.
Simanek AM, Cheng C, Yolken R, Uddin M, Galea S, Aiello AE. Herpesviruses, inflammatory markers and incident depression in a longitudinal study of Detroit residents. Psychoneuroendocrinology. 2014;50:139–48.
Burgdorf KS, Trabjerg BB, Pedersen MG, Nissen J, Banasik K, Pedersen OB, et al. Large-scale study of Toxoplasma and Cytomegalovirus shows an association between infection and serious psychiatric disorders. Brain Behav Immun. 2019;79:152–8.
Frye MA, Coombes BJ, McElroy SL, Jones-Brando L, Bond DJ, Veldic M, et al. Association of Cytomegalovirus and Toxoplasma gondii Antibody Titers With Bipolar Disorder. JAMA Psychiatry. 2019;76:1285–93.
Simanek AM, Zheng C, Yolken R, Haan M, Aiello AE. A Longitudinal Study of the Association Between Persistent Pathogens and Incident Depression Among Older U.S. Latinos. J Gerontol A Biol Sci Med Sci. 2019;74:634–41.
Dickerson F, Origoni A, Schweinfurth LAB, Stallings C, Savage CLG, Sweeney K, et al. Clinical and Serological Predictors of Suicide in Schizophrenia and Major Mood Disorders. J Nerv Ment Dis. 2018;206:173–8.
Jaremka LM, Fagundes CP, Glaser R, Bennett JM, Malarkey WB, Kiecolt-Glaser JK. Loneliness predicts pain, depression, and fatigue: understanding the role of immune dysregulation. Psychoneuroendocrinology. 2013;38:1310–7.
Prossin AR, Yolken RH, Kamali M, Heitzeg MM, Kaplow JB, Coryell WH, et al. Cytomegalovirus Antibody Elevation in Bipolar Disorder: Relation to Elevated Mood States. Neural Plasticity. 2015;2015:939780.
Phillips AC, Carroll D, Khan N, Moss P. Cytomegalovirus is associated with depression and anxiety in older adults. Brain Behav Immun. 2008;22:52–55.
Trzonkowski P, Mysliwska J, Godlewska B, Szmit E, Lukaszuk K, Wieckiewicz J, et al. Immune consequences of the spontaneous pro-inflammatory status in depressed elderly patients. Brain Behav Immun. 2004;18:135–48.
Coryell W, Wilcox H, Evans SJ, Pandey GN, Jones-Brando L, Dickerson F, et al. Latent infection, inflammatory markers and suicide attempt history in depressive disorders. J Affect Disord. 2020;270:97–101.
Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain, Behav, Immun. 2001;15:199–226.
Cole SW. Human social genomics. PLoS Genet. 2014;10:e1004601.
Leday GGR, Vertes PE, Richardson S, Greene JR, Regan T, Khan S, et al. Replicable and Coupled Changes in Innate and Adaptive Immune Gene Expression in Two Case-Control Studies of Blood Microarrays in Major Depressive Disorder. Biol Psychiatry. 2018;83:70–80.
Evans DL, Ten Have TR, Douglas SD, Gettes DR, Morrison M, Chiappini MS, et al. Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. Am J Psychiatry. 2002;159:1752–9.
Irwin MR, Levin MJ, Laudenslager ML, Olmstead R, Lucko A, Lang N, et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infect Dis: Off Publ Infect Dis Soc Am. 2013;56:1085–93.
Afsar B, Elsurer R, Eyileten T, Yilmaz MI, Caglar K. Antibody response following hepatitis B vaccination in dialysis patients: does depression and life quality matter? Vaccine. 2009;27:5865–9.
Ford BN, Yolken RH, Dickerson FB, Teague TK, Irwin MR, Paulus MP et al. Reduced immunity to measles in adults with major depressive disorder. Psychol Med. 2019;49:243–9.
Bochennek K, Allwinn R, Langer R, Becker M, Keppler OT, Klingebiel T, et al. Differential loss of humoral immunity against measles, mumps, rubella and varicella-zoster virus in children treated for cancer. Vaccine. 2014;32:3357–61.
Rocca S, Santilli V, Cotugno N, Concato C, Manno EC, Nocentini G, et al. Waning of vaccine-induced immunity to measles in kidney transplanted children. Med (Baltim). 2016;95:e4738.
Titanji K, De Milito A, Cagigi A, Thorstensson R, Grutzmeier S, Atlas A, et al. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108:1580–7.
Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci USA. 2012;109:5995–9.
Marsland AL, Bachen EA, Cohen S, Rabin B, Manuck SB. Stress, immune reactivity and susceptibility to infectious disease. Physiol Behav. 2002;77:711–6.
Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, et al. Letermovir Prophylaxis for Cytomegalovirus in Hematopoietic-Cell Transplantation. N Engl J Med. 2017;377:2433–44.
Diamond DJ, La Rosa C, Chiuppesi F, Contreras H, Dadwal S, Wussow F, et al. A fifty-year odyssey: prospects for a cytomegalovirus vaccine in transplant and congenital infection. Expert Rev Vaccines. 2018;17:889–911.
Victor TA, Khalsa SS, Simmons WK, Feinstein JS, Savitz J, Aupperle RL, et al. Tulsa 1000: a naturalistic study protocol for multilevel assessment and outcome prediction in a large psychiatric sample. BMJ Open. 2018;8:e016620.
Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PS, et al. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology. 2015;40:463–71.
Ford BN, Yolken RH, Dickerson FB, Teague TK, Irwin MR, Paulus MP, et al. Reduced immunity to measles in adults with major depressive disorder. Psychol Med. 2019;49:243–9.
Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94.
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55.
Cardinale F, Chinnici G, Bramerio M, Mai R, Sartori I, Cossu M, et al. Validation of FreeSurfer-estimated brain cortical thickness: comparison with histologic measurements. Neuroinformatics. 2014;12:535–42.
Iscan Z, Jin TB, Kendrick A, Szeglin B, Lu H, Trivedi M, et al. Test-retest reliability of freesurfer measurements within and between sites: Effects of visual approval process. Hum Brain Mapp. 2015;36:3472–85.
Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar Behav Res. 2011;46:399–424.
Little RJ, Rubin DB. Causal effects in clinical and epidemiological studies via potential outcomes: concepts and analytical approaches. Annu Rev Public Health. 2000;21:121–45.
Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–79.
Thomas L, Li F, Pencina M. Using Propensity Score Methods to Create Target Populations in Observational Clinical Research. JAMA. 2020;323:466–7.
Huffman JE. Examining the current standards for genetic discovery and replication in the era of mega-biobanks. Nat Commun. 2018;9:5054.
VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: introducing the E-Value. Ann Intern Med. 2017;167:268–74.
Kalayjian RC, Cohen ML, Bonomo RA, Flanigan TP. Cytomegalovirus ventriculoencephalitis in AIDS. A syndrome with distinct clinical and pathologic features. Med (Baltim). 1993;72:67–77.
Tozzi L, Garczarek L, Janowitz D, Stein DJ, Wittfeld K, Dobrowolny H, et al. Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: mega-analytic findings from a large multi-site cohort. Psychol Med. 2020;50:1020–31.
Schmaal L, Hibar DP, Samann PG, Hall GB, Baune BT, Jahanshad N, et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2017;22:900–9.
MacDuffie KE, Brown GG, McKenna BS, Liu TT, Meloy MJ, Tawa B, et al. Effects of HIV Infection, methamphetamine dependence and age on cortical thickness, area and volume. Neuroimage Clin. 2018;20:1044–52.
Kallianpur KJ, Kirk GR, Sailasuta N, Valcour V, Shiramizu B, Nakamoto BK, et al. Regional cortical thinning associated with detectable levels of HIV DNA. Cereb Cortex. 2012;22:2065–75.
Jung RE, Segall JM, Grazioplene RG, Qualls C, Sibbitt WL, Roldan CA. Cortical thickness and subcortical gray matter reductions in neuropsychiatric systemic lupus erythematosus. PLoS ONE. 2010;5:e9302.
Niu C, Tan X, Liu X, Han K, Niu M, Xu J, et al. Cortical thickness reductions associate with abnormal resting-state functional connectivity in non-neuropsychiatric systemic lupus erythematosus. Brain Imaging Behav. 2018;12:674–84.
Steenwijk MD, Geurts JJ, Daams M, Tijms BM, Wink AM, Balk LJ, et al. Cortical atrophy patterns in multiple sclerosis are non-random and clinically relevant. Brain. 2016;139:115–26.
Tsagkas C, Chakravarty MM, Gaetano L, Naegelin Y, Amann M, Parmar K, et al. Longitudinal patterns of cortical thinning in multiple sclerosis. Hum Brain Mapp. 2020;41:2198–215.
Jacobs HI, Van Boxtel MP, Jolles J, Verhey FR, Uylings HB. Parietal cortex matters in Alzheimer’s disease: an overview of structural, functional and metabolic findings. Neurosci Biobehav Rev. 2012;36:297–309.
Houenou J, d’Albis MA, Daban C, Hamdani N, Delavest M, Lepine JP, et al. Cytomegalovirus seropositivity and serointensity are associated with hippocampal volume and verbal memory in schizophrenia and bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:142–8.
Marsland AL, Gianaros PJ, Abramowitch SM, Manuck SB, Hariri AR. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64:484–90.
Gianella S, Moser C, Vitomirov A, McKhann A, Layman L, Scott B, et al. Presence of asymptomatic cytomegalovirus and Epstein-Barr virus DNA in blood of persons with HIV starting antiretroviral therapy is associated with non-. AIDS Clin Events AIDS. 2020;34:849–57.
Ford BN, Yolken RH, Aupperle RL, Teague TK, Irwin MR, Paulus MP, et al. Association of Early-Life Stress With Cytomegalovirus Infection in Adults With Major Depressive Disorder. JAMA Psychiatry. 2019;76:545–7.
Dodgeon B, Patalay P, Ploubidis GB, Wiggins RD. Exploring the role of early-life circumstances, abilities and achievements on well-being at age 50 years: evidence from the 1958 British birth cohort study. BMJ Open. 2020;10:e031416.
Mackes NK, Golm D, Sarkar S, Kumsta R, Rutter M, Fairchild G, et al. Early childhood deprivation is associated with alterations in adult brain structure despite subsequent environmental enrichment. Proc Natl Acad Sci USA. 2020;117:641–9.
Yaple ZA, Yu R. Functional and Structural Brain Correlates of Socioeconomic Status. Cereb Cortex. 2020;30:181–96.
Klenerman P, Oxenius A. T cell responses to cytomegalovirus. Nat Rev Immunol. 2016;16:367–77.
Acknowledgements
The authors thank all the research participants and wish to acknowledge the contributions of Brenda Davis, Debbie Neal, Chibing Tan, and Ashlee Taylor from the laboratory of TKT toward the transport, processing, and handling of all blood samples. This work was supported by The William K. Warren Foundation, the National Institute of Mental Health (K01MH096077 and R21MH11387 to JS; R01MH098099 to JB), and the National Institute of General Medical Sciences (P20GM121312 to MPP). The funding sources had no role in the design and conduct of the study: collection, management, analysis, and interpretation of the data; preparation, review, or approval of the paper; and decision to submit the paper for publication.
Tulsa 1000 Investigators
Robin Aupperle1, Jerzy Bodurka1, Yoon-Hee Cha1, Justin Feinstein1, Sahib S. Khalsa1, Martin P. Paulus1, Jonathan Savitz1, Teresa A. Victor1
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
Dr PWH reported receiving support from Merck (donated drug) for a clinical trial of letermovir. All other authors have no conflicts of interest to report.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Members of the Tulsa 1000 Investigators are listed below Acknowledgements.
Supplementary information
Rights and permissions
About this article
Cite this article
Zheng, H., Ford, B.N., Bergamino, M. et al. A hidden menace? Cytomegalovirus infection is associated with reduced cortical gray matter volume in major depressive disorder. Mol Psychiatry 26, 4234–4244 (2021). https://doi.org/10.1038/s41380-020-00932-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-00932-y
This article is cited by
-
Herpesviruses and neuropsychiatric disorders: overlooked adversaries or innocent bystanders?
Neuropsychopharmacology (2024)
-
Aged brain and neuroimmune responses to COVID-19: post-acute sequelae and modulatory effects of behavioral and nutritional interventions
Immunity & Ageing (2023)
-
Cytomegalovirus antibodies are associated with mood disorders, suicide, markers of neuroinflammation, and microglia activation in postmortem brain samples
Molecular Psychiatry (2023)
-
AI-based dimensional neuroimaging system for characterizing heterogeneity in brain structure and function in major depressive disorder: COORDINATE-MDD consortium design and rationale
BMC Psychiatry (2023)
-
Depression, aging, and immunity: implications for COVID-19 vaccine immunogenicity
Immunity & Ageing (2022)