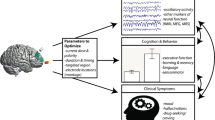
Abstract
Bipolar Disorder is costly and debilitating, and many treatments have side effects. Transcranial Direct Current Stimulation (tDCS) is a well-tolerated neuromodulation technique that may be a useful treatment for Bipolar Disorder if targeted to neural regions implicated in the disorder. One potential region is the left ventrolateral prefrontal cortex (vlPFC), which shows abnormally elevated activity during reward expectancy in individuals with Bipolar Disorder. We used a counterbalanced repeated measures design to assess the impact of cathodal (inhibitory) tDCS over the left vlPFC on reward circuitry activity, functional connectivity, and affect in adults with Bipolar Disorder, as a step toward developing novel interventions for individuals with the disorder. −1mA cathodal tDCS was administered over the left vlPFC versus a control region, left somatosensory cortex, concurrently with neuroimaging. Affect was assessed pre and post scan in remitted Bipolar Disorder (n = 27) and age/gender-matched healthy (n = 31) adults. Relative to cathodal tDCS over the left somatosensory cortex, cathodal tDCS over the left vlPFC lowered reward expectancy-related left ventral striatal activity (F(1,51) = 9.61, p = 0.003), and was associated with lower negative affect post scan, controlling for pre-scan negative affect, (F(1,49) = 5.57, p = 0.02) in all participants. Acute cathodal tDCS over the left vlPFC relative to the left somatosensory cortex reduces reward expectancy-related activity and negative affect post tDCS. Build on these findings, future studies can determine whether chronic cathodal tDCS over the left vlPFC has sustained effects on mood in individuals with Bipolar Disorder, to guide new treatment developments for the disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Individual data along with a data dictionary defining each field are available at the NIMH data archive repository, updates made every 6 months to study end, https://data-archive.nimh.nih.gov/. Following data use certification scientists can access these data.
References
Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS, et al. Grand challenges in global mental health. Nature. 2011;475:27–30.
Meyer B, Johnson SL, Carver CS. Exploring behavioral activation and inhibition sensitivities among college students at risk for bipolar spectrum symptomatology. J Psychopathol Behav Assess. 1999;21:275–92.
Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME, et al. Behavioral approach system and behavioral inhibition system sensitivities and bipolar spectrum disorders: prospective prediction of bipolar mood episodes. Bipolar Disord. 2008;10:310–22.
Giovanelli A, Hoerger M, Johnson SL, Gruber J. Impulsive responses to positive mood and reward are related to mania risk. Cogn Emot. 2013;27:1091–104.
Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: differences between bipolar I and II disorders. Am J Psychiatry. 2013;170:533–41.
Jimura K, Chushak MS, Braver TS. Impulsivity and self-control during intertemporal decision making linked to the neural dynamics of reward value representation. J Neurosci. 2013;33:344–57.
Kim SH, Yoon H, Kim H, Hamann S. Individual differences in sensitivity to reward and punishment and neural activity during reward and avoidance learning. Soc Cogn Affect Neurosci. 2015;10:1219–27.
Singh MK, Kelley RG, Howe ME, Reiss AL, Gotlib IH, Chang KD. Reward processing in healthy offspring of parents with bipolar disorder. JAMA Psychiatry. 2014;71:1148–56.
Bermpohl F, Kahnt T, Dalanay U, Hägele C, Sajonz B, Wegner T, et al. Altered representation of expected value in the orbitofrontal cortex in mania. Hum Brain Mapp. 2010;31:958–69.
Chase HW, Fournier JC, Bertocci MA, Greenberg T, Aslam H, Stiffler R, et al. A pathway linking reward circuitry, impulsive sensation-seeking and risky decision-making in young adults: identifying neural markers for new interventions. Transl Psychiatry. 2017;7:e1096.
Lee SW, O’Doherty JP, Shimojo S. Neural computations mediating one-shot learning in the human brain. PLoS Biol. 2015;13:e1002137.
Smith BJ, Monterosso JR, Wakslak CJ, Bechara A, Read SJ. A meta-analytical review of brain activity associated with intertemporal decisions: Evidence for an anterior-posterior tangibility axis. Neurosci Biobehav Rev. 2018;86:85–98.
Davidson RJ, Shackman AJ, Maxwell JS. Asymmetries in face and brain related to emotion. Trends Cogn Sci. 2004;8:389–91.
Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171:829–43.
Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2011;15:56–67.
Paulus MP, Frank LR. Anterior cingulate activity modulates nonlinear decision weight function of uncertain prospects. Neuroimage. 2006;30:668–77.
Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563.
Weiss SA, Bikson M. Open questions on the mechanisms of neuromodulation with applied and endogenous electric fields. Front Hum Neurosci. 2014;8:227.
Bindman LJ, Lippold OC, Redfearn JW. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. 1964;172:369–82.
Bikson M, Name A, Rahman A. Origins of specificity during tDCS: anatomical, activity-selective, and input-bias mechanisms. Front Hum Neurosci. 2013;7:688.
Dondé C, Neufeld NH, Geoffroy PA. The impact of transcranial direct current stimulation (tdcs) on bipolar depression, mania, and euthymia: a systematic review of preliminary data. Psychiatri Q. 2018;89:855–67.
Loo CK, Alonzo A, Martin D, Mitchell PB, Galvez V, Sachdev P. Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. Br J psychiatry. 2012;200:52–59.
Galvez V, Alonzo A, Martin D, Mitchell PB, Sachdev P, Loo CK. Hypomania induction in a patient with bipolar II disorder by transcranial direct current stimulation (tDCS). J ECT. 2011;27:256–8.
Bai S, Dokos S, Ho KA, Loo C. A computational modelling study of transcranial direct current stimulation montages used in depression. Neuroimage. 2014;87:332–44.
Arul-Anandam AP, Loo C, Mitchell P. Induction of hypomanic episode with transcranial direct current stimulation. J ECT. 2010;26:68–69.
Weber MJ, Messing SB, Rao H, Detre JA, Thompson-Schill SL. Prefrontal transcranial direct current stimulation alters activation and connectivity in cortical and subcortical reward systems: a tDCS-fMRI study. Hum Brain Mapp. 2014;35:3673–86.
Vergallito A, Riva P, Pisoni A, Lauro LJR. Modulation of negative emotions through anodal tDCS over the right ventrolateral prefrontal cortex. Neuropsychologia. 2018;119:128–35
Lang N, Nitsche MA, Paulus W, Rothwell JC, Lemon RN. Effects of transcranial direct current stimulation over the human motor cortex on corticospinal and transcallosal excitability. Exp Brain Res. 2004;156:439–43.
Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol. 2013;591(Pt 7):1987–2000.
Wang Y, Hao Y, Zhou J, Fried PJ, Wang X, Zhang J et al. Direct current stimulation over the human sensorimotor cortex modulates the brain's hemodynamic response to tactile stimulation. Eur J Neurosci. 2015;42:1933–40.
Dmochowski JP, Datta A, Bikson M, Su Y, Parra LC. Optimized multi-electrode stimulation increases focality and intensity at target. J Neural Eng. 2011;8:046011.
Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063.
Bertocci M, Bebko G, Mullin B, Langenecker S, Ladouceur C, Almeida J, et al. Abnormal anterior cingulate cortical activity during emotional n-back task performance distinguishes bipolar from unipolar depressed females. Psychol Med. 2012;42:1417–28.
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9.
McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61:1277–86.
Narum SR. Beyond Bonferroni: less conservative analyses for conservation genetics. Conserv Genet. 2006;7:783–7.
IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.
Tibshirani R. Regression shrinkage and selection via the Lasso. J R Stat Soc Ser B (Methodol). 1996;58:267–88.
Fiori V, Kunz L, Kuhnke P, Marangolo P, Hartwigsen G. Transcranial direct current stimulation (tDCS) facilitates verb learning by altering effective connectivity in the healthy brain. Neuroimage. 2018;181:550–9.
Kessler SK, Turkeltaub PE, Benson JG, Hamilton RH. Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimul. 2012;5:155–62.
Acknowledgements
We would like to acknowledge the participants for their contributions to this study.
Funding
National Institute of Mental Health (MLP, HWC, R21MH108421), Pittsburgh Foundation (MLP). This work was supported by the National Institute of Mental Health (MLP and HWC, grant number R21 MH108421) and the Pittsburgh Foundation (MLP).
Author information
Authors and Affiliations
Contributions
MAB, HWC, and MLP completed the literature search; MAB, BAC, EKE, and MLP created the figures; HWC, BDG, and MLP designed the study; HWC, SG, RS collected the data; MAB, HWC, and RS performed data analysis; MAB, HWC, BAC, BDG, and MLP data interpretation; MAB, HWC, BAC, BDG, and MLP writing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Bertocci, M.A., Chase, H.W., Graur, S. et al. The impact of targeted cathodal transcranial direct current stimulation on reward circuitry and affect in Bipolar Disorder. Mol Psychiatry 26, 4137–4145 (2021). https://doi.org/10.1038/s41380-019-0567-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-019-0567-1
This article is cited by
-
Prefrontal, parietal, and limbic condition-dependent differences in bipolar disorder: a large-scale meta-analysis of functional neuroimaging studies
Molecular Psychiatry (2023)
-
Women with more severe premenstrual syndrome have an enhanced anticipatory reward processing: a magnetoencephalography study
Archives of Women's Mental Health (2023)
-
Brain–computer interface to predict impulse buying behavior using functional near-infrared spectroscopy
Scientific Reports (2022)