Abstract
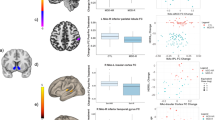
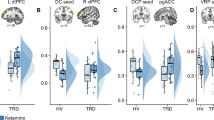
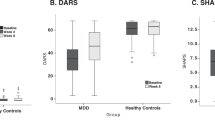
Major depressive disorder (MDD) is a leading cause of disability worldwide, yet current treatment strategies remain limited in their mechanistic diversity. Recent evidence has highlighted a promising novel pharmaceutical target—the KCNQ-type potassium channel—for the treatment of depressive disorders, which may exert a therapeutic effect via functional changes within the brain reward system, including the ventral striatum. The current study assessed the effects of the KCNQ channel opener ezogabine (also known as retigabine) on reward circuitry and clinical symptoms in patients with MDD. Eighteen medication-free individuals with MDD currently in a major depressive episode were enrolled in an open-label study and received ezogabine up to 900 mg/day orally over the course of 10 weeks. Resting-state functional magnetic resonance imaging data were collected at baseline and posttreatment to examine brain reward circuitry. Reward learning was measured using a computerized probabilistic reward task. After treatment with ezogabine, subjects exhibited a significant reduction of depressive symptoms (Montgomery–Asberg Depression Rating Scale score change: −13.7 ± 9.7, p < 0.001, d = 2.08) and anhedonic symptoms (Snaith–Hamilton Pleasure Scale score change: −6.1 ± 5.3, p < 0.001, d = 1.00), which remained significant even after controlling for overall depression severity. Improvement in depression was associated with decreased functional connectivity between the ventral caudate and clusters within the mid-cingulate cortex and posterior cingulate cortex (n = 14, voxel-wise p < 0.005). In addition, a subgroup of patients tested with a probabilistic reward task (n = 9) showed increased reward learning following treatment. These findings highlight the KCNQ-type potassium channel as a promising target for future drug discovery efforts in mood disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJL, et al. Burden of depressive disorders by country, sex, age, and year: findings from the Global Burden of Disease Study 2010. PLoS Med. 2013;10:e1001547.
Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17.
Tollefson GD, Holman S. How long to onset of antidepressant action: a meta-analysis of patients treated with fluoxetine or placebo. Int Clin Psychopharmacol. 1994;9:245–50.
Ferguson JM. SSRI antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin Psychiatry. 2001;3:22–7.
Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–51.
Millan MJ, Goodwin GM, Meyer-Lindenberg A, Ove Ogren S. Learning from the past and looking to the future: emerging perspectives for improving the treatment of psychiatric disorders. Eur Neuropsychopharmacol. 2015;25:599–656.
Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404.
Friedman AK, Juarez B, Ku SM, Zhang H, Calizo RC, Walsh JJ. et al. KCNQ channel openers reverse depressive symptoms via an active resilience mechanism. Nat Commun. 2016;7:11671
Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–6.
Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, et al. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344:313–9.
Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2005;57:319–27.
Han MH, Nestler EJ. Neural substrates of depression and resilience. Neurotherapeutics. 2017;14:677–86.
Connor KM, Davidson JRT. Development of a new resilience scale: the Connor-Davidson Resilience scale (CD-RISC). Depress Anxiety. 2003;18:76–82.
Gunthorpe MJ, Large CH, Sankar R. The mechanism of action of retigabine (ezogabine), a first-in-class K+ channel opener for the treatment of epilepsy. Epilepsia. 2012;53:412–24.
Brodie MJ, Lerche H, Gil-Nagel A, Elger C, Hall S, Shin P, et al. Efficacy and safety of adjunctive ezogabine (retigabine) in refractory partial epilepsy. Neurology. 2010;75:1817–24.
French JA, Abou-Khalil BW, Leroy RF, Yacubian EM, Shin P, Hall S. et al. Randomized, double-blind, placebo-controlled trial of ezogabine (retigabine) in partial epilepsy. Neurology. 2011;76:1555–63.
Stafstrom CE, Grippon S, Kirkpatrick P. Ezogabine (retigabine). Nat Rev Drug Discov. 2011;10:729–30.
Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(SUPPL. 20):22–33.
Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9.
Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone. The Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99–103.
Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–83.
Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry. 2007;4:28–37.
Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J Res Pers. 2006;40:1086–102.
Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, et al. The Columbia-suicide severity rating scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–77.
Brown E, Wood L, Wood S. MedDRA - the medical dictionary for regulatory activities. Drug Saf. 2008;20:109–17.
Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73.
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(SUPPL. 1):208–19.
Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage. 2015;112:267–77.
Jo HJ, Saad ZS, Simmons WK, Milbury LA, Cox RW. Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage. 2010;52:571–82.
Murty VP, Shermohammed M, Smith DV, Carter RMK, Huettel SA, Adcock RA. Resting state networks distinguish human ventral tegmental area from substantia nigra. Neuroimage. 2014;100:580–9.
Barry RL, Coaster M, Rogers BP, Newton AT, Moore J, Anderson AW, et al. On the origins of signal variance in FMRI of the human midbrain at high field. PLoS ONE. 2013;8:1–14.
Eklund A, Nichols TE, Knutsson H. Cluster failure: why fMRI inferences for spatial extent have inflated false-positive rates. Proc Natl Acad Sci. 2016;113:7900–5.
Cox RW, Chen G, Glen DR, Reynolds RC, Taylor PA. FMRI clustering and false positive rates. Proc Natl Acad Sci USA. 2017;114:E3370–1.
Whitton AE, Kakani P, Foti D, Van’T Veer A, Haile A, Crowley DJ, et al. Blunted neural responses to reward in remitted major depression: a high-density event-related potential study. Biol Psychiatry Cogn Neurosci Neuroimaging. 2016;1:87–95.
Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res. 2008;43:76–87.
Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, De Boer P, et al. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry. 2013;73:639–45.
Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–8.
Berton O, Mcclung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the social defeat. Stress. 2008;864:864–9.
Devinsky O, Morrell MJ, Vogt BA. Review article: Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306.
Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22.
Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–67.
Apps MAJ, Rushworth MFS, Chang SWC. The anterior cingulate gyrus and social cognition: tracking the motivation of others. Neuron. 2016;90:692–707.
Jensen J, McIntosh AR, Crawley AP, Mikulis DJ, Remington G, Kapur S. Direct activation of the ventral striatum in anticipation of aversive stimuli. Neuron. 2003;40:1251–7.
Delgado MR, Li J, Schiller D, Phelps EA. The role of the striatum in aversive learning and aversive prediction errors. Philos Trans R Soc B Biol Sci. 2008;363:3787–800.
Brooks AM, Berns GS. Aversive stimuli and loss in the mesocorticolimbic dopamine system. Trends Cogn Sci. 2013;17:281–6.
Quevedo K, Ng R, Scott H, Kodavaganti S, Smyda G, Diwadkar V, et al. Ventral striatum functional connectivity during rewards and losses and symptomatology in depressed patients. Biol Psychol. 2017;123:62–73.
Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–47.
Zhong X, Pu W, Yao S. Functional alterations of fronto-limbic circuit and default mode network systems in first-episode, drug-naïve patients with major depressive disorder: a meta-analysis of resting-state fMRI data. J Affect Disord. 2016;206:280–6.
Hamilton JP, Furman DJ, Chang C, Thomason ME, Dennis E, Gotlib IH. Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry. 2011;70:327–33.
Ho TC, Connolly CG, Henje Blom E, LeWinn KZ, Strigo IA, Paulus MP, et al. Emotion-dependent functional connectivity of the default mode network in adolescent depression. Biol Psychiatry. 2015;78:635–46.
Haber SN, Fudge JL, McFarland NR. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–82.
Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26.
Acknowledgements
We would like to thank the Icahn School of Medicine at Mount Sinai research pharmacists, including Ivy Cohen, Alla Khodzhayeva, and Giuseppe Difiore, for their extensive work on this project.
Funding
Funding for this study was provided by the Friedman Brain Institute and by the Ehrenkranz Laboratory for Human Resilience, both components of the Icahn School of Medicine at Mount Sinai. Additional research support was provided by Doris Duke Charitable Foundation (to JWM) and the National Institute of Mental Health (MH112081, to M-HH; K23MH094707, to JWM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
In the past 5 years, JWM has provided consultation services to Sage Therapeutics, Boehreinger Ingelheim, Novartis, Allergan, Fortress Biotech, Janssen Research and Development, Genentech, MedAvante-ProPhase, and Global Medical Education (GME) and has received research support from Avanir Pharmaceuticals, Inc. JWM is named on a patent pending for neuropeptide Y as a treatment for mood and anxiety disorders. The Icahn School of Medicine (employer of JWM) is named on a patent and has entered into a licensing agreement and will receive payments related to the use of ketamine if it is approved for the treatment of depression. JWM is not named on this patent and will not receive any payments. KCA has received consulting fees from MedAvante-ProPhase for services unrelated to this study. In the past 3 years, DAP has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehreinger Ingelheim, Pfizer, and Posit Science for activities unrelated to the present study. In the past 3 years, DVI has provided consultations to Alkermes, Axsome, MyndAnalytics (CNS Response), Jazz, Lundbeck, Otsuka, and Sunovion and has received research support (through his academic institutions) from Alkermes, Astra Zeneca, Brainsway, LiteCure, Neosync, Roche, and Shire. The other authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Tan, A., Costi, S., Morris, L.S. et al. Effects of the KCNQ channel opener ezogabine on functional connectivity of the ventral striatum and clinical symptoms in patients with major depressive disorder. Mol Psychiatry 25, 1323–1333 (2020). https://doi.org/10.1038/s41380-018-0283-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-018-0283-2
This article is cited by
-
The Potential of KCNQ Potassium Channel Openers as Novel Antidepressants
CNS Drugs (2022)
-
Cooperative synaptic and intrinsic plasticity in a disynaptic limbic circuit drive stress-induced anhedonia and passive coping in mice
Molecular Psychiatry (2021)
-
Advances in depression research: second special issue, 2020, with highlights on biological mechanisms, clinical features, co-morbidity, genetics, imaging, and treatment
Molecular Psychiatry (2020)
-
Advances in depression research: special issue, 2020, with three research articles by Paul Greengard
Molecular Psychiatry (2020)
-
Molecular Psychiatry, August 2020: new impact factor, and highlights of recent advances in psychiatry, including an overview of the brain’s response to stress during infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Molecular Psychiatry (2020)