Abstract
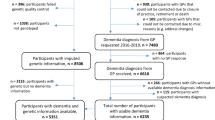
Early identification of younger, non-demented adults at elevated risk for Alzheimer’s disease (AD) is crucial because the pathological process begins decades before dementia onset. Toward that end, we showed that an AD polygenic risk score (PRS) could identify mild cognitive impairment (MCI) in adults who were only in their 50s. Participants were 1176 white, non-Hispanic community-dwelling men of European ancestry in the Vietnam Era Twin Study of Aging (VETSA): 7% with amnestic MCI (aMCI); 4% with non-amnestic MCI (naMCI). Mean age was 56 years, with 89% <60 years old. Diagnosis was based on the Jak-Bondi actuarial/neuropsychological approach. We tested six P-value thresholds (0.05–0.50) for single nucleotide polymorphisms included in the ADPRS. After controlling for non-independence of twins and non-MCI factors that can affect cognition, higher PRSs were associated with significantly greater odds of having aMCI than being cognitively normal (odds ratios (ORs) = 1.36–1.43 for thresholds P < 0.20–0.50). The highest OR for the upper vs. lower quartile of the ADPRS distribution was 3.22. ORs remained significant after accounting for APOE-related SNPs from the ADPRS or directly genotyped APOE. Diabetes was associated with significantly increased odds of having naMCI (ORs = 3.10–3.41 for thresholds P < 0.05–0.50), consistent with naMCI having more vascular/inflammation components than aMCI. Analysis of sensitivity, specificity, and negative and positive predictive values supported some potential of ADPRSs for selecting participants in clinical trials aimed at early intervention. With participants 15+ years younger than most MCI samples, these findings are promising with regard to efforts to more effectively treat or slow AD progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others

References
Golde TE, Schneider LS, Koo EH. Anti-abeta therapeutics in Alzheimer’s disease: the need for a paradigm shift. Neuron. 2011;69:203–13.
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:280–92.
Sperling RA, Jack CR Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3:111cm133.
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011;7:270–9.
Petersen RC, Morris JC. Mild cognitive impairment as a clinical entity and treatment target. Arch Neurol. 2005;62:1160–3.
Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet. 2013;45:1452–8.
Escott-Price V, Sims R, Bannister C, Harold D, Vronskaya M, Majounie E, et al. Common polygenic variation enhances risk prediction for Alzheimer’s disease. Brain. 2015;138:3673–84.
Escott-Price V, Myers AJ, Huentelman M, Hardy J. Polygenic risk score analysis of pathologically confirmed Alzheimer’s disease. Ann Neurol. 2017;82:311–4.
Mormino EC, Sperling RA, Holmes AJ, Buckner RL, De Jager PL, Smoller JW, et al. Polygenic risk of Alzheimer disease is associated with early- and late-life processes. Neurology. 2016;87:481–8.
Tosto G, Bird TD, Tsuang D, Bennett DA, Boeve BF, Cruchaga C, et al. Polygenic risk scores in familial Alzheimer disease. Neurology. 2017;88:1180–6.
Lupton MK, Strike L, Hansell NK, Wen W, Mather KA, Armstrong NJ, et al. The effect of increased genetic risk for Alzheimer’s disease on hippocampal and amygdala volume. Neurobiol Aging. 2016;40:68–77.
Harrison TM, Mahmood Z, Lau EP, Karacozoff AM, Burggren AC, Small GW, et al. An Alzheimer’s disease genetic risk score predicts longitudinal thinning of hippocampal complex subregions in healthy older adults. eNeuro. 2016;3 doi: 10.1523/ENEURO.0098-16.2016.
Sabuncu MR, Buckner RL, Smoller JW, Lee PH, Fischl B, Sperling RA. The association between a polygenic Alzheimer score and cortical thickness in clinically normal subjects. Cereb Cortex. 2012;22:2653–61.
Marioni RE, Campbell A, Hagenaars SP, Nagy R, Amador C, Hayward C, et al. Genetic stratification to identify risk groups for Alzheimer’s disease. J Alzheimers Dis. 2017;57:275–83.
Chauhan G, Adams HH, Bis JC, Weinstein G, Yu L, Toglhofer AM, et al. Association of Alzheimer’s disease GWAS loci with MRI markers of brain aging. Neurobiol Aging. 2015;36:1765 e1767–1716.
Schultz SA, Boots EA, Darst BF, Zetterberg H, Blennow K, Edwards DF, et al. Cardiorespiratory fitness alters the influence of a polygenic risk score on biomarkers of AD. Neurology. 2017;88:1650–8.
Foley SF, Tansey KE, Caseras X, Lancaster T, Bracht T, Parker G, et al. Multimodal brain imaging reveals structural differences in Alzheimer’s disease polygenic risk carriers: a study in healthy young adults. Biol Psychiatry. 2017;81:154–61.
Adams HHH, RFAG deBruijn, Hofman A, Uitterlinden AG, van Duijn CM, Vernooij MW, et al. Genetic risk of neurodegenerative diseases is associated with mild cognitive impairment and conversion to dementia. Alzheimers Dement. 2015;11:1277–85.
Kremen WS, Franz CE, Lyons MJ. VETSA: the Vietnam era twin study of aging. Twin Res Hum Genet. 2013;16:399–402.
Kremen WS, Thompson-Brenner H, Leung YJ, Grant MD, Franz CE, Eisen SA, et al. Genes, environment, and time: the Vietnam era twin study of aging (VETSA). Twin Res Hum Genet. 2006;9:1009–22.
Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis. 2014;42:275–89.
Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17:368–75.
Kremen WS, Jak AJ, Panizzon MS, Spoon KM, Franz CE, Thompson WK, et al. Early identification and heritability of mild cognitive impairment. Int J Epidemiol. 2014;43:600–10.
Edmonds EC, Delano-Wood L, Clark LR, Jak AJ, Nation DA, McDonald CR, et al. Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimers Dement. 2014;11:415–24.
Edmonds EC, Delano-Wood L, Galasko DR, Salmon DP, Bondi MW, Alzheimer’s Disease Neuroimaging Initiative. Subtle cognitive decline and biomarker staging in preclinical Alzheimer’s disease. J Alzheimers Dis. 2015;47:231–42..
Edmonds EC, Delano-Wood L, Jak AJ, Galasko DR, Salmon DP, Bondi MW, et al. “Missed” mild cognitive impairment: High false-negative error rate based on conventional diagnostic criteria. J Alzheimers Dis. 2016;52:685–91.
Delano-Wood L, Bondi MW, Sacco J, Abeles N, Jak AJ, Libon DJ, et al. Heterogeneity in mild cognitive impairment: differences in neuropsychological profile and associated white matter lesion pathology. J Int Neuropsychol Soc. 2009;15:906–14.
Jak AJ, Urban S, McCauley A, Bangen KJ, Delano-Wood L, Corey-Bloom J, et al. Profile of hippocampal volumes and stroke risk varies by neuropsychological definition of mild cognitive impairment. J Int Neuropsychol Soc. 2009;15:890–7.
Wierenga CE, Clark LR, Dev SI, Shin DD, Jurick SM, Rissman RA, et al. Interaction of age and APOE genotype on cerebral blood flow at rest. J Alzheimer’s Dis. 2013;34:921–35.
Wierenga CE, Dev SI, Shin DD, Clark LR, Bangen KJ, Jak AJ, et al. Effect of mild cognitive impairment and APOE genotype on resting cerebral blood flow and its association with cognition. J Cereb Blood Flow Metab. 2012;32:1589–99.
Kremen WS, Moore CS, Franz CE, Panizzon MS, Lyons MJ. Cognition in middle adulthood. In: Finkel D, Reynolds CA, editors. Behavior genetics of cognition across the lifespan. New York: Springer; 2013. pp. 105–34.
Palmer BW, Boone KB, Lesser IM, Wohl MA. Base rates of “impaired” neuropsychological test performance among healthy older adults. Arch Clin Neuropsychol. 1998;13:503–11.
Schoeneborn CA, Heyman KM. Health characteristics of adults aged 55 years and over: United States, 2004–7. Natl Health Stat Report. 2009;16:1–31.
Granholm EL, Panizzon MS, Elman JA, Jak AJ, Hauger RL, Bondi MW, et al. Pupillary responses as a biomarker of early risk for Alzheimer’s disease. J Alzheimer’s Dis. 2017;56:1419–28.
Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94.
Lyons MJ, York TP, Franz CE, Grant MD, Eaves LJ, Jacobson KC, et al. Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychol Sci. 2009;20:1146–52.
Lyons MJ, Panizzon MS, Liu W, McKenzie R, Bluestone NJ, Grant MD, et al. A longitudinal twin study of general cognitive ability over four decades. Dev Psychol. 2017;53:1170–7.
Chen Y, Denny KG, Harvey D, Farias ST, Mungas D, DeCarli C, et al. Progression from normal cognition to mild cognitive impairment in a diverse clinic-based and community-based elderly cohort. Alzheimers Dement. 2016;13:399–405.
Meehl P, Rosen A. Antecedent probability and the efficiency of psychometric signs, patterns, or cutting scores. Psychol Bull. 1955;52:194–216.
Chang CC, Chow CC, Tellier LC, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015;4:1–16.
Chen CY, Pollack S, Hunter DJ, Hirschhorn JN, Kraft P, Price AL. Improved ancestry inference using weights from external reference panels. Bioinformatics. 2013;29:1399–406.
1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526:68–74.
Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet. 2012;44:955–9.
Fuchsberger C, Abecasis GR, Hinds DA. minimac2: faster genotype imputation. Bioinformatics. 2015;31:782–4.
Schultz MR, Lyons MJ, Franz CE, Grant MD, Boake C, Jacobson KC, et al. Apolipoprotein E genotype and memory in the sixth decade of life. Neurology. 2008;70:1771–7.
SAS Institute Inc. SAS OnlineDoc 9.4. Carey, NC: SAS Institute; 2013.
Sadeh N, Spielberg JM, Logue MW, Wolf EJ, Smith AK, Lusk J, et al. SKA2 methylation predicts reduced cortical thickness in prefrontal cortex. Mol Psychiatry. 2016;21:299.
Sadeh N, Wolf EJ, Logue MW, Lusk J, Hayes JP, McGlinchey RE, et al. Polygenic risk for externalizing psychopathology and executive dysfunction in trauma-exposed veterans. Clin Psychol Sci. 2016;4:545–58.
Wolf EJ, Logue MW, Hayes JP, Sadeh N, Schichman SA, Stone A, et al. Accelerated DNA methylation age: associations with PTSD and neural integrity. Psychoneuroendocrinology. 2016;63:155–62.
Li J, Ji L. Adjusting multiple testing in multilocus analyses using the eigenvalues of a correlation matrix. Heredity. 2005;95:221–7.
R Development Core Team. R: a Language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012.
Lobo JM, Jiménez-Valverde A, Real R. AUC: A misleading measure of the performance of predictive distribution models. Glob Ecol Biogeogr. 2008;17:145–51.
Finch CE, Shams S. Apolipoprotein E and sex bias in cerebrovascular aging of men and mice. Trends Neurosci. 2016;39:625–37.
Blacker D, Haines JL, Rodes L, Terwedow H, Go RC, Harrell LE, et al. ApoE-4 and age at onset of Alzheimer’s disease: The NIMH genetics initiative. Neurology. 1997;48:139–47.
Jack CR Jr, Wiste HJ, Weigand SD, Knopman DS, Vemuri P, Mielke MM, et al. Age, sex, and APOE ε4 effects on memory, brain structure, and β-Amyloid across the adult life span. JAMA Neurol. 2015;72:511–9.
Katon W, Pedersen HS, Ribe AR, Fenger-Gron M, Davydow D, Waldorff FB, et al. Effect of depression and diabetes mellitus on the risk for dementia: a national population-based cohort study. JAMA Psychiatry. 2015;72:612–9.
Mez J, Mukherjee S, Thornton T, Fardo DW, Trittschuh E, Sutti S, et al. The executive prominent/memory prominent spectrum in Alzheimer’s disease is highly heritable. Neurobiol Aging. 2016;41:115–21.
Harris SE, Davies G, Luciano M, Payton A, Fox HC, Haggarty P, et al. Polygenic risk for Alzheimer’s disease is not associated with cognitive ability or cognitive aging in non-demented older people. J Alzheimers Dis. 2014;39:565–74.
Acknowledgements
This work was supported by National Institute on Aging R01 AG018386, AG022381, AG022982, AG050595 (W.S.K.), R01 AG018384 (M.J.L.), R03 AG046413 (C.E.F), and K08 AG047903 (M.S.P), and the VA San Diego Center of Excellence for Stress and Mental Health. The content is the responsibility of the authors and does not necessarily represent official views of the NIA, NIH, or VA. The Cooperative Studies Program of the U.S. Department of Veterans Affairs provided financial support for development and maintenance of the Vietnam Era Twin Registry. We would also like to acknowledge the continued cooperation and participation of the members of the VET Registry and their families.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Dale is a Founder of and holds equity in CorTechs Labs, Inc, and serves on its Scientific Advisory Board. He is a member of the Scientific Advisory Board of Human Longevity, Inc. and receives funding through research agreements with General Electric Healthcare and Medtronic, Inc. The terms of these arrangements have been reviewed and approved by UCSD in accordance with its conflict of interest policies. The remaining authors declare that they have no conflict of interest.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Logue, M.W., Panizzon, M.S., Elman, J.A. et al. Use of an Alzheimer’s disease polygenic risk score to identify mild cognitive impairment in adults in their 50s. Mol Psychiatry 24, 421–430 (2019). https://doi.org/10.1038/s41380-018-0030-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-018-0030-8
This article is cited by
-
Impact of genetic predisposition to late-onset neurodegenerative diseases on early life outcomes and brain structure
Translational Psychiatry (2024)
-
A polygenic risk score for Alzheimer’s disease constructed using APOE-region variants has stronger association than APOE alleles with mild cognitive impairment in Hispanic/Latino adults in the U.S.
Alzheimer's Research & Therapy (2023)
-
Functional variants identify sex-specific genes and pathways in Alzheimer’s Disease
Nature Communications (2023)
-
Genetic loci of beta-aminoisobutyric acid are associated with aging-related mild cognitive impairment
Translational Psychiatry (2023)
-
Genetic effects on longitudinal cognitive decline during the early stages of Alzheimer’s disease
Scientific Reports (2021)