Abstract
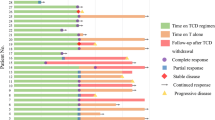
Langerhans cell histiocytosis (LCH) lacks a standardized first-line therapy. This single-center, phase 2 prospective study (NCT04121819) enrolled 61 newly diagnosed adult LCH patients with multisystem or multifocal single system disease from October 2019 to June 2022. Subcutaneous cytarabine (100 mg/m2 for 5 days) was administered in 35-day cycles for 12 total cycles. The primary endpoint was event-free survival (EFS). The median age was 33 years (range 18–66). Twelve patients (19.7%) had liver involvement, of which 2 also had spleen involvement. Among 43 patients undergoing next-generation sequencing, BRAF alterations (44.2%) were most frequent, followed by TP53 (16.3%), MAP2K1 (14.0%) and IDH2 (11.6%). MAPK pathway alterations occurred in 28 patients (65.1%). The overall response rate was 93.4%, with 20 (32.7%) achieving complete response and 37 (60.7%) partial response. After a median 30 months follow-up, 21 (34.4%) relapsed without deaths. Estimated 3-year OS and EFS were 100.0% and 58.5%, respectively. Multivariate analysis identified ≥3 involved organs (P = 0.007; HR 3.937, 95% CI: 1.456–9.804) and baseline lung involvement (P = 0.028; HR 2.976, 95% CI: 1.126–7.874) as poor prognostic factors for EFS. The most common grade 3–4 toxicities were neutropenia (27.9%), thrombocytopenia (1.6%), and nausea (1.6%). In conclusion, cytarabine monotherapy is an effective and safe regimen for newly diagnosed adults, while baseline lung or ≥3 involved organs confers poor prognosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Emile JF, Abla O, Fraitag S, Horne A, Haroche J, Donadieu J, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672–81.
Goyal G, Tazi A, Go RS, Rech KL, Picarsic JL, Vassallo R, et al. International expert consensus recommendations for the diagnosis and treatment of Langerhans cell histiocytosis in adults. Blood. 2022;139:2601–21.
Badalian-Very G, Vergilio JA, Degar BA, MacConaill LE, Brandner B, Calicchio ML, et al. Recurrent BRAF mutations in Langerhans cell histiocytosis. Blood. 2010;116:1919–23.
Chen J, Zhao AL, Duan MH, Cai H, Gao XM, Liu T, et al. Diverse kinase alterations and myeloid-associated mutations in adult histiocytosis. Leukemia. 2022;36:573–6.
Nelson DS, van Halteren A, Quispel WT, van den Bos C, Bovee JV, Patel B, et al. Map2k1 and map3k1 mutations in Langerhans cell histiocytosis. Genes Chromosomes Cancer. 2015;54:361–8.
Tazi A, Lorillon G, Haroche J, Neel A, Dominique S, Aouba A, et al. Vinblastine chemotherapy in adult patients with Langerhans cell histiocytosis: a multicenter retrospective study. Orphanet J Rare Dis. 2017;12:95.
Cantu MA, Lupo PJ, Bilgi M, Hicks MJ, Allen CE, McClain KL. Optimal therapy for adults with Langerhans cell histiocytosis bone lesions. Plos One. 2012;7:e43257.
Goyal G, Abeykoon JP, Hu M, Young JR, Shah MV, Bennani NN, et al. Single-agent cladribine as an effective front-line therapy for adults with Langerhans cell histiocytosis. Am J Hematol. 2021;96:E146–50.
Neel A, Artifoni M, Fontenoy AM, Tessoulin B, Lorillon G, Cohen-Aubart F, et al. Long-term efficacy and safety of 2Cda (cladribine) in extra-pulmonary adult-onset Langerhans cell histiocytosis: analysis of 23 cases from the French Histiocytosis Group and systematic literature review. Br J Haematol. 2020;189:869–78.
Saven A, Burian C. Cladribine activity in adult Langerhans-cell histiocytosis. Blood. 1999;93:4125–30.
Duan MH, Han X, Li J, Zhang W, Zhu TN, Han B, et al. Comparison of vindesine and prednisone and cyclophosphamide, etoposide, vindesine, and prednisone as first-line treatment for adult Langerhans cell histiocytosis: a single-center retrospective study. Leuk Res. 2016;42:43–46.
Cao XX, Li J, Zhao AL, He TH, Gao XM, Cai HC, et al. Methotrexate and cytarabine for adult patients with newly diagnosed Langerhans cell histiocytosis: a single arm, single center, prospective phase 2 study. Am J Hematol. 2020;95:E235–8.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Cao XX, Duan MH, Zhao AL, Cai H, Chen J, Gao XM, et al. Treatment outcomes and prognostic factors of patients with adult Langerhans cell histiocytosis. Am J Hematol. 2022;97:203–8.
Girschikofsky M, Arico M, Castillo D, Chu A, Doberauer C, Fichter J, et al. Management of adult patients with Langerhans cell histiocytosis: recommendations from an expert panel on behalf of Euro-Histio-Net. Orphanet J Rare Dis. 2013;8:72.
Goyal G, Acosta-Medina AA, Abeykoon JP, Dai C, Ravindran A, Vassallo R, et al. Long-term outcomes among adults with Langerhans cell histiocytosis. Blood Adv. 2023;7:6568–78.
Gadner H, Minkov M, Grois N, Potschger U, Thiem E, Arico M, et al. Therapy prolongation improves outcome in multisystem Langerhans cell histiocytosis. Blood. 2013;121:5006–14.
Arico M, Girschikofsky M, Genereau T, Klersy C, McClain K, Grois N, et al. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39:2341–8.
Han HX, Chang L, Lang M, Lin H, Li J, Duan MH, et al. Clinical characteristics, genomic profiling and outcomes of single system multifocal Langerhans cell histiocytosis in adults with bone involvement. Blood Cancer J. 2023;13:135.
Heritier S, Emile JF, Barkaoui MA, Thomas C, Fraitag S, Boudjemaa S, et al. BRAF mutation correlates with high-risk Langerhans cell histiocytosis and increased resistance to first-line therapy. J Clin Oncol. 2016;34:3023–30.
Diamond EL, Durham BH, Ulaner GA, Drill E, Buthorn J, Ki M, et al. Efficacy of MEK inhibition in patients with histiocytic neoplasms. Nature. 2019;567:521–4.
Diamond EL, Subbiah V, Lockhart AC, Blay JY, Puzanov I, Chau I, et al. Vemurafenib for BRAF V600-mutant Erdheim-Chester disease and Langerhans cell histiocytosis: Analysis of data from the histology-independent, phase 2, open-label VE-BASKET study. Jama Oncol. 2018;4:384–8.
Reiner AS, Durham BH, Yabe M, Petrova-Drus K, Francis JH, Rampal RK, et al. Outcomes after interruption of targeted therapy in patients with histiocytic neoplasms. Br J Haematol. 2023;203:389–94.
Acknowledgements
The authors thank all the patients and their families for their trust, respect and support. They also acknowledge all clinicians for their help in accomplishing this work.
Funding
This study was supported by the National Key Research and Development Program of China (2022YFC2705003), Beijing Natural Science Haidian frontier Foundation (L222081), and the National High Level Hospital Clinical Research Funding (2022-PUMCH-A-193).
Author information
Authors and Affiliations
Contributions
LC and ML contributed to data analysis and patient follow-up; MHD and DBZ retrospectively reviewed patient records and contributed to data collection; LC, ML, and XXC wrote the paper; and all authors revised the paper and approved the submitted version.
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Ethical approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all patients included in the study.
Consent for publication
Consent for publication was obtained from all patients included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chang, L., Lang, M., Lin, H. et al. Phase 2 study using low dose cytarabine for adult patients with newly diagnosed Langerhans cell histiocytosis. Leukemia 38, 803–809 (2024). https://doi.org/10.1038/s41375-024-02174-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-024-02174-1