Abstract
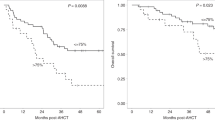
There remains a lack of consensus as to the most appropriate primary therapy in Waldenstrőm macroglobulinemia (WM). We evaluated a novel bortezomib-based combination and developed a sensitive WM-specific flow cytometry assay (limit of detection 0.004% of leucocytes) to assess bone marrow (BM) response. Sixty treatment-naïve WM patients were enroled into this phase II trial and randomised (2:1) to receive cyclophosphamide and rituximab with either bortezomib (BRC) or fludarabine (FCR). The primary objective was to assess the overall response rate (ORR) in eligible patients receiving BRC (N = 41). An ORR of 97.6% (95%CI:87.1–99.9) was observed; 27 (65.9%) patients remain alive without progression after 62.6 months median follow-up, with 2-, 3- and 5-year progression-free survival (PFS) rates of 92.7% (95%CI:79.0–97.6), 80.5% (95%CI:64.8–89.7) and 65.5% (95%CI:48.8–77.9). Persistent WM B-cells were demonstrable in 19/38 patients at the end of treatment (median 0.24%, range 0.02–11.2%). PFS was markedly longer in patients with BM B-cell depletion (<0.004%) compared to those who had persistent BM B-cells detectable at end of treatment (HR = 0.06, 95%CI:0.01–0.47, p < 0.001), and remained independently associated after adjusting for baseline risk stratification or investigator-assessed response. BRC is a tolerable, highly efficacious regimen for treatment-naïve WM patients. BM B-cell depletion is independently associated with patient outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Original data are available for this study upon reasonable request and review. Please contact Rebecca.auer@nhs.net.
References
Kastritis E, Leblond V, Dimopoulos MA, Kimby E, Staber P, Kersten MJ, et al. Waldenström’s macroglobulinaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv41–50.
Castillo JJ, Advani RH, Branagan AR, Buske C, Dimopoulos MA, D’Sa S, et al. Consensus treatment recommendations from the tenth International Workshop for Waldenström Macroglobulinaemia. Lancet Haematol. 2020;7:e827–37.
Pratt G, El-Sharkawi D, Kothari J, D’Sa S, Auer R, McCarthy H, et al. Diagnosis and management of Waldenström macroglobulinaemia-A British Society for Haematology guideline. Br J Haematol. 2022;197:171–87.
Del Giudice I, Matutes E, Osuji N, Parry-Jones N, Swansbury J, Catovsky D. Delayed response to fludarabine in lymphoplasmacytic lymphoma/Waldenström’s macroglobulinemia. Haematologica 2005;90:268–70.
Varghese AM, Rawstron AC, Ashcroft AJ, Moreton P, Owen RG. Assessment of bone marrow response in Waldenström’s macroglobulinemia. Clin Lymphoma Myeloma. 2009;9:53–5.
Treon SP, Ioakimidis L, Soumerai JD, Patterson CJ, Sheehy P, Nelson M, et al. Primary therapy of Waldenstrom macroglobulinemia with bortezomib, dexamethasone, and rituximab: WMCTG clinical trial 05-180. J Clin Oncol. 2009;27:3830–5.
Ghobrial IM, Xie W, Padmanabhan S, Badros A, Rourke M, Leduc R, et al. Phase II trial of weekly bortezomib in combination with rituximab in untreated patients with Waldenstrom Macroglobulinemia. Am J Hematol. 2010;85:670–4.
Treon SP, Yang G, Hanzis C, Ioakimidis L, Verselis SJ, Fox EA, et al. Attainment of complete/very good partial response following rituximab-based therapy is an important determinant to progression-free survival, and is impacted by polymorphisms in FCGR3A in Waldenstrom macroglobulinaemia. Br J Haematol. 2011;154:223–8.
Varettoni M, Zibellini S, Merli M, Drandi D, Jiménez C, Furlan D, et al. Molecular remission is an independent predictor of progression-free survival in patients with Waldenström macroglobulinemia treated with chemo-immunotherapy: results from the FIL_BIOWM study. Hematol Oncol. 2023;41:574–77.
Fürstenau M, De Silva N, Eichhorst B, Hallek M. Minimal residual disease assessment in CLL: ready for use in clinical routine? Hemasphere. 2019;3:e287.
Munshi NC, Avet-Loiseau H, Anderson KC, Neri P, Paiva B, Samur M, et al. A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma. Blood Adv. 2020;4:5988–99.
Owen RG, Kyle RA, Stone MJ, Rawstron AC, Leblond V, Merlini G, et al. VIth International Workshop on Waldenström macroglobulinaemia. Response assessment in Waldenström macroglobulinaemia: update from the VIth International Workshop. Br J Haematol. 2013;160:171–6.
Sargent DJ, Chan V, Goldberg RM. A three-outcome design for phase II clinical trials. Control Clin Trials. 2001;22:117–25.
Paiva B, Corchete LA, Vidriales MB, Garcia-Sanz R, Perez JJ, Aires-Mejia I, et al. The cellular origin and malignant transformation of Waldenström macroglobulinemia. Blood. 2015;125:2370–80.
Rummel MJ, Niederle N, Maschmeyer G, Banat GA, von Grunhagen U, Losem C, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet. 2013;381:1203–10.
Laribi K, Poulain S, Willems L, Merabet F, Le Calloch R, Eveillard JR, et al. Bendamustine plus rituximab in newly-diagnosed Waldenström macroglobulinaemia patients. A study on behalf of the French Innovative Leukaemia Organization (FILO). Br J Haematol. 2019;186:146–9.
Leleu X, Soumerai J, Roccaro A, Hatjiharissi E, Hunter ZR, Manning R, et al. Increased incidence of transformation and myelodysplasia/acute leukemia in patients with Waldenstrom macroglobulinemia treated with nucleoside analogs. J Clin Oncol. 2009;27:250–5.
Buske C, Dimopoulos MA, Grunenberg A, Kastritis E, Tomowiak C, Mahe B, et al. Bortezomib in combination with Dexamethasone, Rituximab and Cyclophosphamide (B-DRC) as first-line treatment of Waldenström’s macroglobulinaemia: results of a prospectively randomized multicenter European phase II trial. J Clin Oncol. 2023;41:2607–16.
García-Sanz R, Ocio E, Caballero A, Magalhaes RJ, Alonso J, Lopes-Anglada L, et al. Post-treatment bone marrow residual disease > 5% by flow cytometry is highly predictive of short progression-free and overall survival in patients with Waldenström’s macroglobulinemia. Clin Lymphoma Myeloma Leuk 2011;11:168–71.
Xu L, Hunter ZR, Yang G, Zhou Y, Cao Y, Liu X, et al. MYD88 L265P in Waldenström macroglobulinemia, immunoglobulin M monoclonal gammopathy, and other B-cell lymphoproliferative disorders using conventional and quantitative allele-specific polymerase chain reaction. Blood. 2013;121:2051–8.
Drandi D, Genuardi E, Dogliotti I, Ferrante M, Jiménez C, Guerrini F, et al. Highly sensitive MYD88L265P mutation detection by droplet digital polymerase chain reaction in Waldenström macroglobulinemia. Haematologica. 2018;103:1029–37.
Moreno DF, Lopez-Guerra M, Paz S, Oliver-Caldes A, Mena M-P, Correa JG, et al. Prognostic impact of MYD88 and CXCR4 mutations assessed by droplet digital polymerase chain reaction in IgM monoclonal gammopathy of undetermined significance and smouldering Waldenström macroglobulinaemia. Br J Haematol. 2023;200:187–96.
Acknowledgements
This study was supported by the grant C26303/A14139 to RA from Cancer Research UK and by Roche Products Ltd and Janssen-Cilag Ltd. The authors would like to thank Professor Malcolm Ranson (Christie Hospital NHS Trust), Dr Robert Glynne-Jones (Mount Vernon Hospital), Professor Peter Donnan (University of Dundee), Professor Claire Harrison (Guy’s and St Thomas’ NHS Foundation Trust), Professor David Miles (Mount Vernon Cancer Centre), Dr Paul Silcocks (University of Liverpool) and Dr Caroline Kelly (CR UK Clinical Trials Unit, Glasgow) for providing study oversight as the Independent Data Monitoring Committee (current and former members) as well as all of the patients and investigators who contributed to this study.
Author information
Authors and Affiliations
Contributions
RdT was responsible for designing the laboratory flow cytometry assay, performing laboratory analyses, verifying and analysing data, interpreting results, making the figures and writing parts of the paper. NC was responsible for designing the trial, verifying and analysing data, interpreting results, making the figures and writing parts of the paper. LCH was responsible for designing the trial and contributed to writing the paper. SD’S was responsible for designing the trial and performing the research by treating patients and collecting their data. GP was responsible for designing the trial and performing the research by treating patients and collecting their data. GC was responsible for performing the research by treating patients and collecting their data. LC was responsible for performing laboratory analyses. RS was responsible for performing laboratory analyses and analysing the data. WT was responsible for designing the trial and helped with writing of the trial protocol. BP was responsible for designing the trial and helped with writing of the trial protocol. PS was responsible for designing the trial. OS was responsible for collecting the trial data. RO was responsible for responsible for designing the trial, performing the research by treating patients and collecting their data, interpreting results and writing parts of the paper. RA was responsible for designing the trial, performing the research by treating patients and collecting their data, interpreting results and writing the paper. As Chief Investigator of the trial she had overall responsibility for the trial and trial protocol. All authors had full access to all the data in the study, critically reviewed the manuscript and approved the content. All authors accept responsibility to submit for publication.
Corresponding author
Ethics declarations
Competing interests
LC-H has received funding (which in part pays staff salary) to Sponsor and coordinate clinical trials. Funding has been awarded/used in the last 2 years from Millennium pharmaceutics inc., Bristol-Myers Squibb Pharmaceuticals Ltd., Amgen GmBbH., Celgene Ltd., Merck Sharp and Dohme Ltd., Janssen-Cilag Ltd and Pfizer Ltd., SD’S has received research funds from BeiGene and Janssen-Cilag Ltd. and has acted on an advisory board and received honoraria from BeiGene, Janssen-Cilag Ltd and Sanofi, WT has received honoraria and consultancy fees from Roche, Gilead and Celgene, GC has received educational grants from Novartis and Bristol-Myers Squibb, RO has received honoraria from AstraZeneca and Celgene and has acted on an advisory board and received honoraria from BeiGene and Janssen-Cilag Ltd, RA has received consultancy fees from BeiGene and Janssen-Cilag Ltd. Funding has been awarded to support a clinical trial from Janssen-Cilag Ltd. No other authors declared conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
de Tute, R., Counsell, N., Clifton-Hadley, L. et al. Long-term outcomes by bone marrow B-cell depletion from the R2W trial of bortezomib with cyclophosphamide and rituximab in Waldenstrőm macroglobulinaemia. Leukemia 38, 822–828 (2024). https://doi.org/10.1038/s41375-024-02162-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-024-02162-5