Abstract
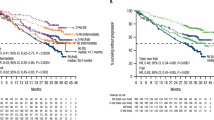
Osteonecrosis is a significant toxicity of acute lymphoblastic leukemia (ALL) therapy. In retrospective analyses, superior event-free survival was noted among affected adolescents in an earlier trial. We prospectively assessed osteonecrosis incidence, characteristics, and risk factors in patients 1–30 years with newly diagnosed high-risk B-ALL on COG AALL0232. Patients were randomized to induction dexamethasone vs prednisone, and interim maintenance high-dose methotrexate vs escalating-dose Capizzi methotrexate/pegaspargase. Event-free and overall survival were compared between patients with/without imaging-confirmed osteonecrosis. Osteonecrosis developed in 322/2730 eligible, evaluable patients. The 5-year cumulative incidence was 12.2%. Risk was greater in patients ≥10 years (hazard ratio [HR], 7.23; P < 0.0001), particularly females (HR, 1.37; P = 0.0057), but lower in those with asparaginase allergy (HR, 0.60; P = 0.0077). Among rapid early responders ≥10 years, risk was greater with dexamethasone (HR, 1.84; P = 0.0003) and with prednisone/Capizzi (HR, 1.45; P = 0.044), even though neither therapy was independently associated with improved survival. Patients with osteonecrosis had higher 5-year event-free (HR, 0.51; P < 0.0001) and overall survival (HR, 0.42; P < 0.0001), and this was directly attributable to reduced relapse rates (HR, 0.57; P = 0.0014). Osteonecrosis in high-risk B-ALL patients is associated with improved survival, suggesting an important role for host factors in mediating both toxicity and enhanced efficacy of specific therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The COG Data Sharing policy describes the release and use of COG individual subject data for use in research projects in accordance with National Clinical Trials Network (NCTN) Program and NCI Community Oncology Research Program (NCORP) Guidelines. Only data expressly released from the oversight of the relevant COG Data and Safety Monitoring Committee (DSMC) are available to be shared. Data are available to researchers who wish to analyze the data in secondary studies to enhance the public health benefit of the original work and agree to the terms and conditions of use. Requests for access to COG protocol research data should be sent to: datarequest@childrensoncologygroup.org. Data are available to researchers whose proposed analysis is found by COG to be feasible and of scientific merit and who agree to the terms and conditions of use. For all requests, no other study documents, including the protocol, will be made available and no end date exists for requests.
References
Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. New Engl J Med. 2015;373:1541–52.
Kunstreich M, Kummer S, Laws HJ, Borkhardt A, Kuhlen M. Osteonecrosis in children with acute lymphoblastic leukemia. Haematologica. 2016;101:1295–305.
Mattano LA Jr, Devidas M, Nachman JB, Sather HN, Hunger SP, Steinherz PG, et al. Effect of alternate-week versus continuous dexamethasone scheduling on the risk of osteonecrosis in paediatric patients with acute lymphoblastic leukaemia: results from the CCG-1961 randomised cohort trial. Lancet Oncol. 2012;13:906–15.
Mostoufi-Moab S, Halton J. Bone morbidity in childhood leukemia: epidemiology, mechanisms, diagnosis, and treatment. Curr Osteoporos Rep. 2014;12:300–12.
Niinimäki T, Harila-Saari A, Niinimäki R. The diagnosis and classification of osteonecrosis in patients with childhood leukemia. Pediatr Blood Cancer. 2015;62:198–203.
Parasole R, Valsecchi MG, Silvestri D, Locatelli F, Barisone E, Petruzziello F, et al. Correspondence: osteonecrosis in childhood acute lymphoblastic leukemia: a retrospective cohort study of the Italian Association of Pediatric Haemato-Oncology (AIEOP). Blood Cancer J. 2018;8:115.
Badhiwala JH, Nayiager T, Athale UH. The development of thromboembolism may increase the risk of osteonecrosis in children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2015;62:1851–4.
Finkelstein Y, Blonquist TM, Vijayanathan V, Stevenson KE, Neuberg DS, Silverman LB, et al. A thymidylate synthase polymorphism is associated with increased risk for bone toxicity among children treated for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2017;64:e26393.
Inaba H, Pui CH. Glucocorticoid use in acute lymphoblastic leukaemia. Lancet Oncol. 2010;11:1096–106.
Liu C, Kawedia JD, Cheng C, Pei D, Fernandez CA, Cai X, et al. Clinical utility and implications of asparaginase antibodies in acute lymphoblastic leukemia. Leukemia. 2012;26:2303–9.
Relling MV, Yang W, Das S, Cook EH, Rosner GL, Neel M, et al. Pharmacogenetic risk factors for osteonecrosis of the hip among children with leukemia. J Clin Oncol. 2004;22:3930–6.
Albertsen BK, Grell K, Abrahamsson J, Lund B, Vettenranta K, Jónsson ÓG, et al. Intermittent versus continuous PEG-asparaginase to reduce asparaginase-associated toxicities: a NOPHO ALL2008 randomized study. J Clin Oncol. 2019;37:1638–46.
Schrappe M, Bleckmann K, Zimmermann M, Biondi A, Möricke A, Locatelli F, et al. Reduced-intensity delayed intensification in standard-risk pediatric acute lymphoblastic leukemia defined by undetectable minimal residual disease: results of an international randomized trial (AIEOP-BFM ALL 2000). J Clin Oncol. 2018;36:244–53.
Finch ER, Smith CA, Yang W, Liu Y, Kornegay NM, Panetta JC, et al. Asparaginase formulation impacts hypertriglyceridemia during therapy for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2020;67:e28040.
Janke LJ, Van Driest SL, Portera MV, Atreya RV, Denny JC, Pei D, et al. Letter: Hypertension is a modifiable risk factor for osteonecrosis in acute lymphoblastic leukemia. Blood. 2019;134:983–6.
Kawedia JD, Kaste SC, Pei D, Panetta JC, Cai X, Cheng C, et al. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2011;117:2340–7.
Mattano LA Jr, Sather HN, Trigg ME, Nachman JB. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children’s Cancer Group. J Clin Oncol. 2000;18:3262–72.
Mogensen SS, Harila-Saari A, Mäkitie O, Myrberg IH, Niinimäki R, Vestli A, et al. Comparing osteonecrosis clinical phenotype, timing, and risk factors in children and young adults treated for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2018;65:e27300.
Niinimäki RA, Harila-Saari AH, Jartti AE, Seuri RM, Riikonen PV, Pääkkö EL, et al. High body mass index increases the risk for osteonecrosis in children with acute lymphoblastic leukemia. J Clin Oncol. 2007;25:1498–504.
Sakamoto K, Imamura T, Kihira K, Suzuki K, Ishida H, Morita H, et al. Low incidence of osteonecrosis in childhood acute lymphoblastic leukemia treated with ALL-97 and ALL-02 study of Japan Association of Childhood Leukemia Study Group. J Clin Oncol. 2018;36:900–7.
te Winkel ML, Pieters R, Hop WCJ, de Groot-Kruseman HA, Lequin MH, van der Sluis IM, et al. Prospective study on incidence, risk factors, and long-term outcome of osteonecrosis in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2011;29:4143–50.
Yao S, Zhu Q, Cole PD, Stevenson K, Harris MH, Schultz E, et al. Genetic ancestry and skeletal toxicities among childhood acute lymphoblastic leukemia patients in the DFCI 05-001 cohort. Blood Adv. 2021;5:451–8.
French D, Hamilton LH, Mattano LA Jr, Sather HN, Devidas M, Nachman JB, et al. A PAI-1 (SERPINE1) polymorphism predicts osteonecrosis in children with acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2008;111:4496–9.
Karol SE, Yang W, Van Driest SL, Chang TY, Kaste S, Bowton E, et al. Genetics of glucocorticoid-associated osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2015;126:1770–6.
Ramsey LB, Pounds S, Cheng C, Cao X, Yang W, Smith C, et al. Genetics of pleiotropic effects of dexamethasone. Pharmacogenet Genomics. 2017;27:294–302.
Yang W, Devidas M, Liu Y, Smith C, Dai Y, Winick N, et al. Letter: Genetics of osteonecrosis in pediatric acute lymphoblastic leukemia and general populations. Blood. 2021;137:1550–2.
Valtis YK, Stevenson KE, Place AE, Silverman LB, Vrooman LM, Gotti G, et al. Orthopedic toxicities among adolescents and young adults treated on DFCI ALL Consortium trials. Blood Adv. 2022;6:72–81.
Seibel NL, Steinherz PG, Sather HN, Nachman JB, DeLaat C, Ettinger LJ, et al. Early postinduction intensification therapy improves survival for children and adolescents with high-risk acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Blood. 2008;111:2548–55.
Larsen EC, Devidas M, Chen S, Salzer WL, Raetz EA, Loh ML, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: a report from Children’s Oncology Group study AALL0232. J Clin Oncol. 2016;34:2380–8.
Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39.
Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Statist. 1988;16:1141–54.
Lynggaard LS, Rank CU, Hansen SN, Højfeldt SG, Henriksen LT, Jarvis KB, et al. Asparaginase enzyme activity levels and toxicity in childhood acute lymphoblastic leukemia: a NOPHO ALL2008 study. Blood Adv. 2022;6:138–47.
Yang L, Panetta JC, Cai X, Yang W, Pei D, Cheng C, et al. Asparaginase may influence dexamethasone pharmacokinetics in acute lymphoblastic leukemia. J Clin Oncol. 2008;26:1932–9.
Chen S-H, Pei D, Yang W, Cheng C, Jeha S, Cox NJ, et al. Genetic variations in GRIA1 on chromosome 5q33 related to asparaginase hypersensitivity. Clin Pharmacol Ther. 2010;88:191–6.
Ito C, Evans WE, McNinch L, Coustan-Smith E, Mahmoud H, Pui CH, et al. Comparative cytotoxicity of dexamethasone and prednisolone in childhood acute lymphoblastic leukemia. J Clin Oncol. 1996;14:2370–6.
Möricke A, Zimmermann M, Valsecchi MG, Stanulla M, Biondi A, Mann G, et al. Dexamethasone vs prednisone in induction treatment of pediatric ALL: results of the randomized trial AIEOP-BFM ALL 2000. Blood. 2016;127:2101–12.
Teuffel O, Kuster SP, Hunger SP, Conter V, Hitzler J, Ethier MC, et al. Dexamethasone versus prednisone for induction therapy in childhood acute lymphoblastic leukemia: a systematic review and meta-analysis. Leukemia. 2011;25:1232–8.
Henriksen LT, Nersting J, Raja RA, Frandsen TL, Rosthøj S, Schrøder H, et al. Cerebrospinal fluid asparagine depletion during pegylated asparaginase therapy in children with acute lymphoblastic leukaemia. Br J Haematol. 2014;166:213–20.
Salzer WL, Burke MJ, Devidas M, Dai Y, Hardy KK, Kairalla JA, et al. Impact of intrathecal triple therapy versus intrathecal methotrexate on disease-free survival for high-risk B-lymphoblastic leukemia: Children’s Oncology Group study AALL1131. J Clin Oncol. 2020;38:2628–38.
Acknowledgements
This work was supported by COG Chair’s Operations grants U10 CA98543 and U10 CA180886, COG Statistics and Data Center grants U10 CA098413 and U10 CA180899, and St. Baldrick’s Foundation funding. The study is registered at ClinicalTrials.gov, number NCT00075725. MLL is an Endowed Professor of Pediatric Cancer Research, The Aldarra Foundation Endowed Chair, Bill and June Boeing, Founders. EAR is the Kids of NYU Foundation Professor at NYU Langone Health. SPH is the Jeffrey E. Perelman Distinguished Chair in Pediatrics at The Children’s Hospital of Philadelphia. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
All authors participated in protocol development and data collection. LAM, MD, MLL, YD, ZC, and SPH analyzed the data and wrote the draft manuscript. All authors reviewed the paper, provided input on content and interpretation of results, and approved the final version. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Competing interests
LAM has received consulting fees from Novartis and Pfizer, and owns stock in Pfizer. MLL has received consulting fees from Medsix Therapeutics. SPH owns stock in Amgen, and has received honoraria from Amgen and Servier, and consulting fees from Novartis. The other authors declare no competing financial interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mattano, L.A., Devidas, M., Loh, M.L. et al. Development of osteonecrosis and improved survival in B-ALL: results of Children’s Oncology Group Trial AALL0232. Leukemia 38, 258–265 (2024). https://doi.org/10.1038/s41375-023-02099-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-023-02099-1