Abstract
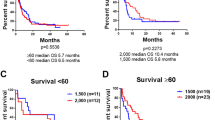
Prior experience indicated that use of higher doses of cytarabine during induction for acute myeloid leukemia (AML) with a histone deacetylase inhibitor resulted in high response rates. S1203 was a randomized multicenter trial for previously untreated patients aged 18–60 with AML which compared daunorubicin and cytarabine (DA), idarubicin with higher dose cytarabine (IA) and IA with vorinostat (IA + V). The primary endpoint was event free survival (EFS). 738 patients were randomized: 261 to each DA and IA arms and 216 to the IA + V arm. 96, 456, and 150 patients had favorable-, intermediate-, and unfavorable-risk cytogenetics, respectively. 152 were NPM1 and 158 FLT3 mutated. The overall remission rate was 77.5% including 62.5% CR and 15.0% CRi. No differences in remission, EFS, or overall survival were observed among the 3 arms except for the favorable cytogenetics subset who had improved outcomes with DA and postremission high dose cytarabine. A trend towards increased toxicity was observed with the IA and IA + V arms. The use of higher dose cytarabine during induction therapy in younger patients with AML, with or without vorinostat, does not result in improved outcomes. (Funded by the US National Institutes of Health and others, ClinicalTrials.gov number, NCT01802333.)
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets analyzed for the current study will be available through the NCTN data archive within 6 months of publication: https://nctn-data-archive.nci.nih.gov.
References
Dohner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia. N. Engl J Med. 2015;373:1136–52.
Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N. Engl J Med. 2012;366:1079–89.
Dohner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77.
Dinardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl J Med. 2020;383:617–29.
Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N. Engl J Med. 1994;331:896–903.
Fernandez HF, Sun Z, Yao X, Litzow MR, Luger SM, Paietta EM, et al. Anthracycline dose intensification in acute myeloid leukemia. N. Engl J Med. 2009;361:1249–59.
Garcia-Manero G, Tambaro FP, Bekele NB, Yang H, Ravandi F, Jabbour E, et al. Phase II trial of vorinostat with idarubicin and cytarabine for patients with newly diagnosed acute myelogenous leukemia or myelodysplastic syndrome. J Clin Oncol. 2012;30:2204–10.
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl J Med. 2017;377:454–64.
Holowiecki J, Grosicki S, Giebel S, Robak T, Kyrcz-Krzemien S, Kuliczkowski K, et al. Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: a multicenter, randomized phase III study. J Clin Oncol. 2012;30:2441–8.
Garcia-Manero G, Yang H, Bueso-Ramos C, Ferrajoli A, Cortes J, Wierda WG, et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndromes. Blood. 2008;111:1060–6.
Kadia TM, Yang H, Ferrajoli A, Maddipotti S, Schroeder C, Madden TL, et al. A phase I study of vorinostat in combination with idarubicin in relapsed or refractory leukaemia. Br J Haematol. 2010;150:72–82.
Sanchez-Gonzalez B, Yang H, Bueso-Ramos C, Hoshino K, Quintas-Cardama A, Richon VM, et al. Antileukemia activity of the combination of an anthracycline with a histone deacetylase inhibitor. Blood. 2006;108:1174–82.
Pagel JM, Othus M, Garcia-Manero G, Fang M, Radich JP, Rizzieri DA, et al. Rapid Donor Identification Improves Survival in High-Risk First-Remission Patients With Acute Myeloid Leukemia. JCO Oncol Pract. 2020;16:e464–75.
Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–83.
Medeiros BC, Kohrt HE, Arber DA, Bangs CD, Cherry AM, Majeti R, et al. Immunophenotypic features of acute myeloid leukemia with inv(3)(q21q26.2)/t(3;3)(q21;q26.2). Leuk Res. 2010;34:594–7.
Fang M, Storer B, Estey E, Othus M, Zhang L, Sandmaier BM, et al. Outcome of patients with acute myeloid leukemia with monosomal karyotype who undergo hematopoietic cell transplantation. Blood. 2011;118:1490–4.
Middeke JM, Fang M, Cornelissen JJ, Mohr B, Appelbaum FR, Stadler M, et al. Outcome of patients with abnl(17p) acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. Blood. 2014;123:2960–7.
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9.
Appelbaum FR, Bernstein ID. Gemtuzumab ozogamicin for acute myeloid leukemia. Blood. 2017;130:2373–6.
Acknowledgements
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers CA180888, CA180819, CA18020, CA180821, CA180863, CA077202, CA180816, CA180855, CA180846, CA189848, CA189957, CA180858, CA189860, CA180798, CA189953, CA189856, CA180835, CA189822, CA189971, CA189858, CA189830, CA180801, CA189853, CA189872, CA180826, CA11083, CA46282, CA46368, CA46136, CA46113, CA16385, CA12644, CA04919, CA13612, CA35119, CA73590, CA58723, CA016672, and CCSRI #021039. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the patients who participated in this trial and their families, the team members from each treating institution, and the support staff of the SWOG Operations Office and Statistics and Data Management Center.
Author information
Authors and Affiliations
Contributions
GGM, HPE, MO, JMP, JPR, and FRA designed the study and wrote the protocol. NAP, DAR, GM, SAS, MRL, MLS, BCM, MAS, TLL, GLU, BLP, JEK, RAL, RMS, DC, JE, SML, SRM, AM, HPE conducted the study. MF did cytogenetics review. MO and AM conducted all statistical analyses. GGM, HPE, and MO wrote the first draft of the manuscript. All authors reviewed the results of statistical analysis, participated in the interpretation of the results, and critically revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Garcia-Manero, G., Podoltsev, N.A., Othus, M. et al. A randomized phase III study of standard versus high-dose cytarabine with or without vorinostat for AML. Leukemia 38, 58–66 (2024). https://doi.org/10.1038/s41375-023-02073-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-023-02073-x