Abstract
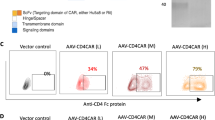
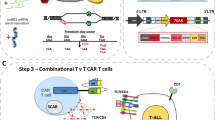
CAR-T therapies to treat T-cell malignancies face unique hurdles. Normal and malignant T cells usually express the same target for CAR, leading to fratricide. CAR-T cells targeting CD7, which is expressed in various malignant T cells, have limited expansion due to fratricide. Using CRISPR/Cas9 to knockout CD7 can reduce the fratricide. Here we developed a 2-in-1 strategy to insert EF1α-driven CD7-specific CAR at the disrupted CD7 locus and compared it to two other known strategies: one was random integration of CAR by a retrovirus and the other was site-specific integration at T-cell receptor alpha constant (TRAC) locus, both in the context of CD7 disruption. All three types of CD7 CAR-T cells with reduced fratricide could expand well and displayed potent cytotoxicity to both CD7+ tumor cell lines and patient-derived primary tumors. Moreover, EF1α-driven CAR expressed at the CD7 locus enhances tumor rejection in a mouse xenograft model of T-cell acute lymphoblastic leukemia (T-ALL), suggesting great clinical application potential. Additionally, this 2-in-1 strategy was adopted to generate CD7-specific CAR-NK cells as NK also expresses CD7, which would prevent contamination from malignant cells. Thus, our synchronized antigen-knockout CAR-knockin strategy could reduce the fratricide and enhance anti-tumor activity, advancing clinical CAR-T treatment of T-cell malignancies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
June CH, Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379:64–73.
Park JH, Riviere I, Gonen M, Wang X, Senechal B, Curran KJ, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med. 2018;378:449–59.
Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-cell therapy bb2121 in relapsed or refractory multiple myeloma. N Engl J Med. 2019;380:1726–37.
Fleischer LC, Spencer HT, Raikar SS. Targeting T cell malignancies using CAR-based immunotherapy: challenges and potential solutions. J Hematol Oncol. 2019;12:141.
Ware RE, Scearce RM, Dietz MA, Starmer CF, Palker TJ, Haynes BF. Characterization of the surface topography and putative tertiary structure of the human CD7 molecule. J Immunol. 1989;143:3632–40.
Wenzinger C, Williams E, Gru AA. Updates in the pathology of precursor lymphoid neoplasms in the revised fourth edition of the who classification of tumors of hematopoietic and lymphoid tissues. Curr Hematol Malig Rep. 2018;13:275–88.
Baum W, Steininger H, Bair HJ, Becker W, Hansen-Hagge TE, Kressel M, et al. Therapy with CD7 monoclonal antibody TH‐69 is highly effective for xenografted human. Br J Haematol. 1996;95:327–38.
Pauza ME, Doumbia SO, Pennell CA. Construction and characterization of human CD7-specific single-chain Fv immunotoxins. J Immunol. 1997;158:3259–69.
Uckun FM, Ramakrishnan S, Houston LL. Immunotoxin-mediated elimination of clonogenic tumor cells in the presence of human bone marrow. J Immunol. 1985;134:2010–6.
Zhang J, Jain A, Milhas S, Williamson DJ, Mysliwy J, Lodge A, et al. An antibody-drug conjugate with intracellular drug release properties showing specific cytotoxicity against CD7-positive cells. Leuk Res. 2021;108:106626.
Yu Y, Li J, Zhu X, Tang X, Bao Y, Sun X, et al. Humanized CD7 nanobody-based immunotoxins exhibit promising anti-T-cell acute lymphoblastic leukemia potential. Int J Nanomed. 2017;12:1969–83.
Brammer JE, Saliba RM, Jorgensen JL, Ledesma C, Gaballa S, Poon M, et al. Multi-center analysis of the effect of T-cell acute lymphoblastic leukemia subtype and minimal residual disease on allogeneic stem cell transplantation outcomes. Bone Marrow Transpl. 2017;52:20–7.
Litzow MR, Ferrando AA. How I treat T-cell acute lymphoblastic leukemia in adults. Blood. 2015;126:833–41.
Bayon-Calderon F, Toribio ML, Gonzalez-Garcia S. Facts and challenges in immunotherapy for T-cell acute lymphoblastic leukemia. Int J Mol Sci. 2020;21:7685.
U.S. National Library of Medicine. ClinicalTrials. https://clinicaltrials.gov.
Pan J, Tan Y, Wang G, Deng B, Ling Z, Song W, et al. Donor-derived CD7 chimeric antigen receptor T cells for T-cell acute lymphoblastic leukemia: first-in-human, phase I trial. J Clin Oncol. 2021;39:3340–52.
Teachey DT, Hunger SP. Anti-CD7 CAR T cells for T-ALL: impressive early-stage efficacy. Nat Rev Clin Oncol. 2021;18:677–8.
Gomes-Silva D, Srinivasan M, Sharma S, Lee CM, Wagner DL, Davis TH, et al. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood 2017;130:285–96.
Mamonkin M, Mukherjee M, Srinivasan M, Sharma S, Gomes-Silva D, Mo F, et al. Reversible transgene expression reduces fratricide and permits 4-1BB costimulation of CAR T cells directed to T-cell malignancies. Cancer Immunol Res. 2018;6:47–58.
Rasaiyaah J, Georgiadis C, Preece R, Mock U, Qasim W. TCRalphabeta/CD3 disruption enables CD3-specific antileukemic T cell immunotherapy. JCI Insight. 2018;3:e99442.
Cooper ML, Choi J, Staser K, Ritchey JK, Devenport JM, Eckardt K, et al. An “off-the-shelf” fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies. Leukemia 2018;32:1970–83.
Georgiadis C, Rasaiyaah J, Gkazi SA, Preece R, Etuk A, Christi A, et al. Base-edited CAR T cells for combinational therapy against T cell malignancies. Leukemia. 2021;35:3466–81.
Gomes-Silva D, Atilla E, Atilla PA, Mo F, Tashiro H, Srinivasan M, et al. CD7 CAR T Cells for the therapy of acute myeloid leukemia. Mol Ther. 2019;27:272–80.
Png YT, Vinanica N, Kamiya T, Shimasaki N, Coustan-Smith E, Campana D. Blockade of CD7 expression in T cells for effective chimeric antigen receptor targeting of T-cell malignancies. Blood Adv. 2017;1:2348–60.
Freiwan A, Zoine J, Crawford JC, Vaidya A, Schattgen SA, Myers J, et al. Engineering naturally occurring CD7 negative T cells for the immunotherapy of hematological malignancies. Blood 2022;140:2684–96.
Wang X, Riviere I. Clinical manufacturing of CAR T cells: foundation of a promising therapy. Mol Ther Oncol. 2016;3:16015.
Ellis DJ. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum Gene Ther. 2005;16:1241–6.
Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJ, Hamieh M, Cunanan KM, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–7.
Melenhorst JJ, Chen GM, Wang M, Porter DL, Chen C, Collins MA, et al. Decade-long leukaemia remissions with persistence of CD4(+) CAR T cells. Nature. 2022;602:503–9.
Mayer KE, Mall S, Yusufi N, Gosmann D, Steiger K, Russelli L, et al. T-cell functionality testing is highly relevant to developing novel immuno-tracers monitoring T cells in the context of immunotherapies and revealed CD7 as an attractive target. Theranostics. 2018;8:6070–87.
Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, et al. A human memory T cell subset with stem cell-like properties. Nat Med. 2011;17:1290–7.
Gu Y, Zhang J, Ma X, Kim BW, Wang H, Li J, et al. Stabilization of the c-Myc protein by CAMKIIgamma promotes T cell lymphoma. Cancer Cell. 2017;32:115–28.
Lu P, Liu Y, Yang J, Zhang X, Yang X, Wang H, et al. Naturally selected CD7 CAR-T therapy without genetic manipulations for T-ALL/LBL: first-in-human phase 1 clinical trial. Blood. 2022;140:321–34.
Pan J, Tan Y, Wang G, Deng B, Ling Z, Song W, et al. Donor-derived CD7 chimeric antigen receptor T cells for T-cell acute lymphoblastic leukemia: first-in-human, phase I trial. J Clin Oncol. 2021;39:3340–51.
Tan Y, Shan L, Zhao L, Deng B, Ling Z, Zhang Y, et al. Long-term follow-up of donor-derived CD7 CAR T-cell therapy in patients with T-cell acute lymphoblastic leukemia. J Hematol Oncol. 2023;16:34.
Duan Y, Chen R, Huang Y, Meng X, Chen J, Liao C, et al. Tuning the ignition of CAR: optimizing the affinity of scFv to improve CAR-T therapy. Cell Mol Life Sci. 2021;79:14.
Xiao Q, Zhang X, Tu L, Cao J, Hinrichs CS, Su X. Size-dependent activation of CAR-T cells. Sci Immunol. 2022;7:eabl3995.
Depil S, Duchateau P, Grupp SA, Mufti G, Poirot L. ‘Off-the-shelf’ allogeneic CAR T cells: development and challenges. Nat Rev Drug Discov. 2020;19:185–99.
Acknowledgements
The authors thank the support of Zhejiang Provincial Key Laboratory of Immunity and Inflammatory Diseases, and thank Yueting Xin, Chun Guo, Jiajia Wang, Yingying Huang from the core facilities, Zhejiang University School of Medicine for their technical support.
Funding
This research was funded by the National Key R&D Program of China 2021YFA0909900 (JS), 2018YFA0800102 (YG), the National Natural Science Foundation of China grants 31971324 (JS), 31970555 (YG), 82161138028 (JS), 81973993 (XG), Zhejiang Provincial Natural Science Foundation grant LR20H160003 (JS), LR20C070001 (XG) and the Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang grant 2020R01006 (JS, YG).
Author information
Authors and Affiliations
Contributions
JJ and JS designed the studies and conceived the experiments. JJ, JC, and YW performed most of the experiments; CL, DY, KS conducted data analysis; YH, YT, and XG contributed reagents. JJ, YG, and JS wrote the manuscript. YG and JS supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal studies were reviewed and approved by the Ethical Committee of Westlake University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, J., Chen, J., Liao, C. et al. Inserting EF1α-driven CD7-specific CAR at CD7 locus reduces fratricide and enhances tumor rejection. Leukemia 37, 1660–1670 (2023). https://doi.org/10.1038/s41375-023-01948-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-023-01948-3
This article is cited by
-
CRISPR–Cas9 applications in T cells and adoptive T cell therapies
Cellular & Molecular Biology Letters (2024)
-
Development of NK cell-based cancer immunotherapies through receptor engineering
Cellular & Molecular Immunology (2024)