Abstract
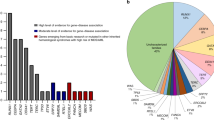
Broader genetic screening has led to the growing recognition of the role of germline variants associated with adult bone marrow failure (BMF) and myeloid neoplasia (MN) not exclusively in children and young adults. In this study, we applied a germline variant panel to 3008 adult BMF and MN cases to assess the importance of germline genetics and its impact on disease phenotype and prognosis. In our cohort, up to 9.7% of BMF and 5.3% of MN cases carried germline variants. Our cohort also included heterozygous carriers of recessive traits, suggesting they contribute to the risk of BMF and MN. By gene category, variants of Fanconi anemia gene family represented the highest-frequency category for both BMF and MN cases, found in 4.9% and 1.7% cases, respectively. In addition, about 1.4% of BMF and 0.19% of MN cases harbored multiple germline variants affecting often functionally related genes as compound heterozygous. The burden of germline variants in BMF and MN was clearly associated with acquisition of monosomy 7. While BMF cases carrying germline variants showed similar overall survival as compared to the wild-type (WT) cases, MN cases with germline variants experienced a significantly shorter overall survival as compared to WT cases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All the data that support the findings of this study are available within the Article and Supplementary Files. NGS targeted samples can be requested from the Cleveland Clinic by contacting the corresponding author (maciejj@ccf.org). Whole genome sequencing data can be requested from the Munich Leukemia laboratory (torsten.haferlach@mll.com).
References
Keel SB, Scott A, Sanchez-Bonilla M, Ho PA, Gulsuner S, Pritchard CC, et al. Genetic features of myelodysplastic syndrome and aplastic anemia in pediatric and young adult patients. Haematologica. 2016;101:1343–50.
Feurstein S, Drazer M, Godley LA. Germline predisposition to hematopoietic malignancies. Hum Mol Genet. 2021;30:R225–35.
Rio-Machin A, Vulliamy T, Hug N, Walne A, Tawana K, Cardoso S, et al. The complex genetic landscape of familial MDS and AML reveals pathogenic germline variants. Nat Commun. 2020;11:1044.
Feurstein S, Churpek JE, Walsh T, Keel S, Hakkarainen M, Schroeder T, et al. Germline variants drive myelodysplastic syndrome in young adults. Leukemia. 2021;35:2439–44.
Ramus SJ, Song H, Dicks E, Tyrer JP, Rosenthal AN, Intermaggio MP, et al. Germline mutations in the BRIP1, BARD1, PALB2, and NBN genes in women with ovarian cancer. J Natl Cancer Inst. 2015;107:djv214.
Tischkowitz M, Xia B. PALB2/FANCN: recombining cancer and Fanconi anemia. Cancer Res. 2010;70:7353–9.
Reilly CR, Myllymäki M, Redd R, Padmanaban S, Karunakaran D, Tesmer V, et al. The clinical and functional effects of TERT variants in myelodysplastic syndrome. Blood. 2021;138:898–11.
Fenwarth L, Caulier A, Lachaier E, Goursaud L, Marceau-Renaut A, Fournier E, et al. Hereditary predisposition to acute myeloid leukemia in older adults. Hemasphere. 2021;5:e552.
Kim B, Yun W, Lee ST, Choi JR, Yoo KH, Koo HH, et al. Prevalence and clinical implications of germline predisposition gene mutations in patients with acute myeloid leukemia. Sci Rep. 2020;10:14297.
Przychodzen B, Makishima H, Sekeres MA, Balasubramanian SK, Thota S, Patel BJ, et al. Fanconi Anemia germline variants as susceptibility factors in aplastic anemia, MDS and AML. Oncotarget. 2018;9:2050–57.
Li ST, Wang J, Wei R, Shi R, Adema V, Nagata Y, et al. Rare germline variant contributions to myeloid malignancy susceptibility. Leukemia. 2020;34:1675–78.
Gurnari C, Pagliuca S, Patel BJ, Awada H, Kongkiatkamon S, Terkawi L, et al. Implication of PIGA genotype on erythrocytes phenotype in Paroxysmal Nocturnal Hemoglobinuria. Leukemia. 2021;35:2431–34.
Gurnari C, Graham AC, Efanov A, Pagliuca S, Durrani J, Awada H, et al. Frequency and perturbations of various peripheral blood cell populations before and after eculizumab treatment in paroxysmal nocturnal hemoglobinuria. Blood Cells Mol Dis. 2021;87:102528.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–74.
Tyner JW, Tognon CE, Bottomly D, Wilmot B, Kurtz SE, Savage SL, et al. Functional genomic landscape of acute myeloid leukaemia. Nature. 2018;562:526–31.
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–21.
Yang F, Long N, Anekpuritanang T, Bottomly D, Savage JC, Lee T, et al. Identification and prioritization of myeloid malignancy germline variants in a large cohort of adult AML patients. Blood. 2022;139:1208–21.
Gurnari C, Wahida A, Pagliuca S, Durmaz A, Zawit M, Haferlach T, et al. A study of Telomerase Reverse Transcriptase rare variants in myeloid neoplasia. Hematol Oncol. 2022;40:812–7.
Adema V, Palomo L, Walter W, Mallo M, Hutter S, La Framboise T, et al. Pathophysiologic and clinical implications of molecular profiles resultant from deletion 5q. EBioMedicine. 2022;80:104059.
Kongkiatkamon S, Pagliuca S, Adema V, Nagata Y, Kerr CM, Walter W, et al. Molecular characterization of the histone acetyltransferase CREBBP/EP300 genes in myeloid neoplasia. Leukemia. 2022;36:1185–88.
Forbes SA, Tang G, Bindal N, Bamford S, Dawson E, Cole C, et al. COSMIC (the Catalogue of Somatic Mutations in Cancer): a resource to investigate acquired mutations in human cancer. Nucleic Acids Res. 2010;38:D652–57.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29:24–26.
Markiewicz W, Amikam S, Roguin N, Riss E. Changing haemodynamics in patient with papillary muscle dysfunction. Br Heart J. 1975;37:445–48.
Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, et al. A global reference for human genetic variation. Nature. 2015;526:68–74.
Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla S, et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018;46:D1062–67.
Gurnari C, Pagliuca S, Prata PH, Galimard JE, Catto LFB, Larcher L, et al. Clinical and molecular determinants of clonal evolution in aplastic anemia and paroxysmal nocturnal hemoglobinuria. J Clin Oncol. 2022;JCO2200710. https://doi.org/10.1200/JCO.22.00710. Online ahead of print.
Kopanos C, Tsiolkas V, Kouris A, Chapple CE, Albarca Aguilera M, Meyer R, et al. VarSome: the human genomic variant search engine. Bioinformatics. 2019;35:1978–80.
Carnicer MJ, Lasa A, Buschbeck M, Serrano E, Carricondo M, Brunet S, et al. K313dup is a recurrent CEBPA mutation in de novo acute myeloid leukemia (AML). Ann Hematol. 2008;87:819–27.
Pezeshki A, Podder S, Kamel R, Corey SJ. Monosomy 7/del (7q) in inherited bone marrow failure syndromes: a systematic review. Pediatr Blood Cancer. 2017;64:e26714.
Narumi S, Amano N, Ishii T, Katsumata N, Muroya K, Adachi M, et al. SAMD9 mutations cause a novel multisystem disorder, MIRAGE syndrome, and are associated with loss of chromosome 7. Nat Genet. 2016;48:792–97.
Sahoo SS, Pastor VB, Goodings C, Voss RK, Kozyra EJ, Szvetnik A, et al. Clinical evolution, genetic landscape and trajectories of clonal hematopoiesis in SAMD9/SAMD9L syndromes. Nat Med. 2021;27:1806–17.
Sébert M, Passet M, Raimbault A, Rahmé R, Raffoux E, Sicre de Fontbrune F, et al. Germline DDX41 mutations define a significant entity within adult MDS/AML patients. Blood. 2019;134:1441–44.
Voso MT, Gurnari C. Have we reached a molecular era in myelodysplastic syndromes? Hematol Am Soc Hematol Educ Program. 2021;2021:418–27.
Acknowledgements
We thank The Torsten Haferlach Leukämiediagnostik Stiftung to support this work. The authors also thank The Cancer Genome Atlas, The Beat AML Master Trial, and The German-Austrian Study Group for data accessibility.
Funding
This work was supported by an R35HL135795 (to JPM) and The Leukemia & Lymphoma Society TRP Award 6645-22 (to JPM). SF receives funding from the Olympia Morata Program of the Medical Faculty Heidelberg.
Author information
Authors and Affiliations
Contributions
Authorship contributions are as followed: YK designed the study, analyzed and interpreted the data, and wrote the manuscript; MZ, JD, WS analyzed sequencing data and participated in the original data collection; WSB, TK, BP, MM collected clinical data and edited the manuscript; CG, MAS, TLaF, VV provided important feedback to the manuscript; SF, LAG gave expert opinion of variants assessment and edited the manuscript; M.Meggendorfer, TH shared whole genome sequencing data and edited the manuscript; JPM conceived the study, interpreted the data and wrote the manuscript. All authors participated in discussions and interpretation of the data and results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kubota, Y., Zawit, M., Durrani, J. et al. Significance of hereditary gene alterations for the pathogenesis of adult bone marrow failure versus myeloid neoplasia. Leukemia 36, 2827–2834 (2022). https://doi.org/10.1038/s41375-022-01729-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01729-4