Abstract
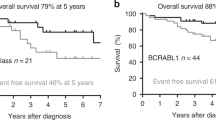
Recently, we defined “CML-like” subtype of BCR::ABL1-positive acute lymphoblastic leukemia (ALL), resembling lymphoid blast crisis of chronic myeloid leukemia (CML). Here we retrospectively analyzed prognostic relevance of minimal residual disease (MRD) and other features in 147 children with BCR::ABL1-positive ALL (diagnosed I/2000–IV/2021, treated according to EsPhALL (n = 133) or other (n = 14) protocols), using DNA-based monitoring of BCR::ABL1 genomic breakpoint and clonal immunoglobulin/T-cell receptor gene rearrangements. Although overall prognosis of CML-like (n = 48) and typical ALL (n = 99) was similar (5-year-EFS 60% and 49%, respectively; 5-year-OS 75% and 73%, respectively), typical ALL presented more relapses while CML-like patients more often died in the first remission. Prognostic role of MRD was significant in the typical ALL (p = 0.0005 in multivariate analysis for EFS). In contrast, in CML-like patients MRD was not significant (p values > 0.2) and inapplicable for therapy adjustment. Moreover, in the typical ALL, risk-prediction could be further improved by considering initial hyperleukocytosis. Early distinguishing typical BCR::ABL1-positive ALL and CML-like patients is essential to enable optimal treatment approach in upcoming protocols. For the typical ALL, tyrosine-kinase inhibitors and concurrent chemotherapy with risk-directed intensity should be recommended; in the CML-like disease, no relevant prognostic feature applicable for therapy tailoring was found so far.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hunger SP, Raetz EA, Loh ML, Mullighan CG. Improving outcomes for high-risk ALL: translating new discoveries into clinical care. Pediatr Blood Cancer. 2011;56:984–93.
Suttorp M, Millot F, Sembill S, Deutsch H, Metzler M. Definition, epidemiology, pathophysiology, and essential criteria for diagnosis of pediatric chronic myeloid leukemia. Cancers. 2021;13:798.
Hijiya N, Suttorp M. How I treat chronic myeloid leukemia in children and adolescents. Blood. 2019;133:2374–84.
Cross NC, White HE, Colomer D, Ehrencrona H, Foroni L, Gottardi E, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015;29:999–1003.
Cazzaniga G, De Lorenzo P, Alten J, Rottgers S, Hancock J, Saha V, et al. Predictive value of minimal residual disease in Philadelphia-chromosome-positive acute lymphoblastic leukemia treated with imatinib in the European intergroup study of post-induction treatment of Philadelphia-chromosome-positive acute lymphoblastic leukemia, based on immunoglobulin/T-cell receptor and BCR/ABL1 methodologies. Haematologica. 2018;103:107–15.
van der Velden VH, Cazzaniga G, Schrauder A, Hancock J, Bader P, Panzer-Grumayer ER, et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: guidelines for interpretation of real-time quantitative PCR data. Leukemia. 2007;21:604–11.
Ravandi F, Jorgensen JL, Thomas DA, O’Brien S, Garris R, Faderl S, et al. Detection of MRD may predict the outcome of patients with Philadelphia chromosome-positive ALL treated with tyrosine kinase inhibitors plus chemotherapy. Blood. 2013;122:1214–21.
Conter V, Bartram CR, Valsecchi MG, Schrauder A, Panzer-Grumayer R, Moricke A, et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115:3206–14.
Hovorkova L, Zaliova M, Venn NC, Bleckmann K, Trkova M, Potuckova E, et al. Monitoring of childhood ALL using BCR-ABL1 genomic breakpoints identifies a subgroup with CML-like biology. Blood. 2017;129:2771–81.
Zaliova M, Fronkova E, Krejcikova K, Muzikova K, Mejstrikova E, Stary J, et al. Quantification of fusion transcript reveals a subgroup with distinct biological properties and predicts relapse in BCR/ABL-positive ALL: implications for residual disease monitoring. Leukemia. 2009;23:944–51.
Biondi A, Gandemer V, De Lorenzo P, Cario G, Campbell M, Castor A, et al. Imatinib treatment of paediatric Philadelphia chromosome-positive acute lymphoblastic leukaemia (EsPhALL2010): a prospective, intergroup, open-label, single-arm clinical trial. Lancet Haematol. 2018;5:e641–52.
Biondi A, Schrappe M, De Lorenzo P, Castor A, Lucchini G, Gandemer V, et al. Imatinib after induction for treatment of children and adolescents with Philadelphia-chromosome-positive acute lymphoblastic leukaemia (EsPhALL): a randomised, open-label, intergroup study. Lancet Oncol. 2012;13:936–45.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70.
van der Veer A, Zaliova M, Mottadelli F, De Lorenzo P, Te Kronnie G, Harrison CJ, et al. IKZF1 status as a prognostic feature in BCR-ABL1-positive childhood ALL. Blood. 2014;123:1691–8.
Smith M, Arthur D, Camitta B, Carroll AJ, Crist W, Gaynon P, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24.
Biondi A, Cario G, De Lorenzo P, Castor A, Conter V, Leoni V, et al. Long-term follow up of pediatric Philadelphia positive acute lymphoblastic leukemia treated with the EsPhALL2004 study: high white blood cell count at diagnosis is the strongest prognostic factor. Haematologica. 2019;104:e13–6.
Acknowledgements
This study was supported by grants from the Czech Health Research Council (NU21-03-00128) and Charles University (UNCE 204012), by the project (Ministry of Health, Czech Republic) for conceptual development of research organization 00064203 (University Hospital Motol, Prague, Czech Republic), by the project National Institute for Cancer Research (Program EXCELES, ID Project No. LX22NPO5102)—Funded by the European Union—Next Generation EU, by Deutsche Krebshilfe (70112958), by the Italian Association for Cancer Research (AIRC) to AB (IG2017—20564) and G. Cazzaniga (IG2015—17593), and by the Comitato Maria Letizia Verga.
Author information
Authors and Affiliations
Contributions
JZ, G.Cario, GCazzaniga, and MZ designed the study; LH, JK, AK, MB, LW, EF, JA, RK, CE, LB, MT, and JStu analyzed samples and provided data; MZ, PDL, MGV, VC, JSta, MS, AB, and JT provided diagnostic and clinical data; all authors participated on data integration, interpretation, and presentation; JZ and MZ wrote the draft; and all authors revised the draft and contributed to the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zuna, J., Hovorkova, L., Krotka, J. et al. Minimal residual disease in BCR::ABL1-positive acute lymphoblastic leukemia: different significance in typical ALL and in CML-like disease. Leukemia 36, 2793–2801 (2022). https://doi.org/10.1038/s41375-022-01668-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-022-01668-0