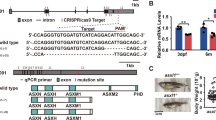
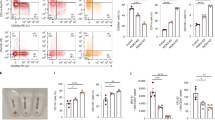
Abstract
TET2 loss-of-function mutations are recurrent events in a wide range of hematological malignancies and a physiologic occurrence in blood cells of healthy older adults. It is currently unknown what determines if a person harboring a somatic TET2 mutation will progress to myelodysplastic syndrome or acute myeloid leukemia. Here we develop a zebrafish tet2 mutant through which we show that tet2 loss leads to restricted hematopoietic differentiation combined with a modest upregulation of p53, which is also characteristic of many inherited bone marrow failure syndromes. Uniquely in the context of emergency hematopoiesis by external stimuli, such as infection or cytokine stimulation, lack of tet2 leads hematopoietic stem cells to undergo excessive proliferation, resulting in an accumulation of immature cells, which are poised to become leukemogenic following additional genetic/epigenetic perturbations. This same phenomenon observed in zebrafish extends to human hematopoietic stem cells, identifying TET2 as a critical relay switch in the context of stress hematopoiesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data and materials availability
All sequencing data have been deposited into GEO repository RNAseq (GSE136349) and meDIP/hmeDIP data (GSE136761). The codes used for meDIP-seq/hmeDIP and RNAseq analysis are uploaded to the GitHub page (https://github.com/vinothkr11/zebrafishtet2). The plasmid used for mRNA synthesis pCS2+ gcsf (Addgene ID:132947) is made available through Addgene.
References
Wu H, Zhang Y. Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation. Genes Dev. 2011;25:2436–52.
Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia.2013;28:241.
Chou W-C, Chou S-C, Liu C-Y, Chen C-Y, Hou H-A, Kuo Y-Y, et al. TET2 mutation is an unfavorable prognostic factor in acute myeloid leukemia patients with intermediate-risk cytogenetics. Blood.2011;118:3803–10.
Genovese G, Kähler AK, Handsaker RE, Lindberg J, Rose SA, Bakhoum SF, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N. Engl J Med. 2014;371:2477–87.
Young AL, Challen GA, Birmann BM, Druley TE. Clonal haematopoiesis harbouring AML-associated mutations is ubiquitous in healthy adults. Nat Commun.2016;7:12484.
Chen L-L, Lin H-P, Zhou W-J, He C-X, Zhang Z-Y, Cheng Z-L, et al. SNIP1 recruits TET2 to regulate c-MYC target genes and cellular DNA damage response. Cell Rep. 2018;25:1485–500. e4
Rasmussen KD, Berest I, Keβler S, Nishimura K, Simón-Carrasco L, Vassiliou GS, et al. TET2 binding to enhancers facilitates transcription factor recruitment in hematopoietic cells. Genome Res. 2019;29:564–75.
Elghetany MT, Alter BP. p53 protein overexpression in bone marrow biopsies of patients with shwachman-diamond syndrome has a prevalence similar to that of patients with refractory anemia. Arch Pathol Lab Med. 2002;126:452–5.
Xu H, Xiao T, Chen C-H, Li W, Meyer CA, Wu Q, et al. Sequence determinants of improved CRISPR sgRNA design. J Genome Res. 2015;25:1147–57.
Prykhozhij SV, Fuller C, Steele SL, Veinotte CJ, Razaghi B, Robitaille JM, et al. Optimized knock-in of point mutations in zebrafish using CRISPR/Cas9. Nucleic Acids Res. 2018;46:e102–e.
Bennett CM, Kanki JP, Rhodes J, Liu TX, Paw BH, Kieran MW, et al. Myelopoiesis in the zebrafish, Danio rerio. Blood.2001;98:643–51.
Lauter G, Söll I, Hauptmann G. Two-color fluorescent in situ hybridization in the embryonic zebrafish brain using differential detection systems. BMC Developmental Biol. 2011;11:43.
LeBlanc J, Venezia Bowman T, Zon L. Transplantation of whole kidney marrow in adult zebrafish. JoVE. 2007;2:159.
Metelo AM, Noonan HR, Li X, Jin Y, Baker R, Kamentsky L, et al. Pharmacological HIF2α inhibition improves VHL disease–associated phenotypes in zebrafish model. J Clin Investig. 2015;125:1987–97.
Brunetti L, Gundry MC, Kitano A, Nakada D, Goodell MA. Highly efficient gene disruption of murine and human hematopoietic progenitor cells by CRISPR/Cas9. JoVE. 2018;134:57278.
Nishikawa T, Ota T, Isogai T. Prediction whether a human cDNA sequence contains initiation codon by combining statistical information and similarity with protein sequences. Bioinformatics.2000;16:960–7.
Wu D, Hu D, Chen H, Shi G, Fetahu IS, Wu F, et al. Glucose-regulated phosphorylation of TET2 by AMPK reveals a pathway linking diabetes to cancer. Nature.2018;559:637–41.
El-Brolosy MA, Kontarakis Z, Rossi A, Kuenne C, Günther S, Fukuda N, et al. Genetic compensation triggered by mutant mRNA degradation. Nature.2019;568:193–7.
Sommer C, Straehle C, Koethe U, Hamprecht FA, editors. Ilastik: Interactive learning and segmentation toolkit. 2011 IEEE international symposium on biomedical imaging: From nano to macro; 2011: IEEE.
Magnusson M, Brun ACM, Miyake N, Larsson J, Ehinger M, Bjornsson JM, et al. HOXA10 is a critical regulator for hematopoietic stem cells and erythroid/megakaryocyte development. Blood.2007;109:3687–96.
Ciganda M, Williams N. Eukaryotic 5S rRNA biogenesis. Wiley Interdiscip Rev: Rna. 2011;2:523–33.
Hatakeyama S. TRIM family proteins: roles in autophagy, immunity, and carcinogenesis. Trends Biochemical Sci. 2017;42:297–311.
Dror Y. p53 protein overexpression in shwachman-diamond syndrome. Arch Pathol Lab Med. 2002;126:1157–8.
Jiang D, Wei S, Chen F, Zhang Y, Li J. TET3-mediated DNA oxidation promotes ATR-dependent DNA damage response. EMBO Rep. 2017;18:781–96.
Gartel AL, Tyner AL. Transcriptional regulation of the p21(WAF1/CIP1) gene. Exp Cell Res. 1999;246:280–9.
Martin C, Ohayon D, Alkan M, Mocek J, Pederzoli-Ribeil M, Candalh C, et al. Neutrophil-expressed p21/waf1 favors inflammation resolution in pseudomonas aeruginosa infection. Am J Respiratory Cell Mol Biol. 2016;54:740–50.
Steinman RA, Huang J, Yaroslavskiy B, Goff JP, Ball ED, Nguyen A. Regulation of p21 (WAF1) expression during normal myeloid differentiation. Blood, J Am Soc Hematol. 1998;91:4531–42.
Singh RP, Grinenko T, Ramasz B, Franke K, Lesche M, Dahl A, et al. Hematopoietic stem cells but not multipotent progenitors drive erythropoiesis during chronic erythroid stress in epo transgenic mice. Stem Cell Rep. 2018;10:1908–19.
Lundberg P, Karow A, Nienhold R, Looser R, Hao-Shen H, Nissen I, et al. Clonal evolution and clinical correlates of somatic mutations in myeloproliferative neoplasms. Blood.2014;123:2220–8.
Gjini E, Mansour MR, Sander JD, Moritz N, Nguyen AT, Kesarsing M, et al. A Zebrafish Model of myelodysplastic syndrome produced through tet2 genomic editing. Mol Cell Biol. 2015;35:789–804.
Ge L, Zhang RP, Wan F, Guo DY, Wang P, Xiang LX, et al. TET2 plays an essential role in erythropoiesis by regulating lineage-specific genes via DNA oxidative demethylation in a Zebrafish Model. Mol Cell Biol. 2014;34:989–1002.
Ko M, Bandukwala HS, An J, Lamperti ED, Thompson EC, Hastie R, et al. Ten-Eleven-Translocation 2 (TET2) negatively regulates homeostasis and differentiation of hematopoietic stem cells in mice. Proc Natl Acad Sci. 2011;108:14566–71.
Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N. Engl J Med. 2017;377:111–21.
Huang Y, Zhang J, Liu J, Hu Y, Ni S, Yang Y, et al. Fish TRIM35 negatively regulates the interferon signaling pathway in response to grouper nodavirus infection. Fish Shellfish Immunol. 2017;69:142–52.
Wang Y, Yan S, Yang B, Wang Y, Zhou H, Lian Q, et al. TRIM35 negatively regulates TLR7- and TLR9-mediated type I interferon production by targeting IRF7. FEBS Lett. 2015;589:1322–30.
Ahlstedt J, Wang Y, Fang Y. A rare case of myelodysplastic syndrome with ring sideroblasts, SF3B1 and TET2 mutations in a patient with beta thalassemia trait. North Am J Med Sci. 2017;10:32–5.
Ponnikorn S, Kong SP, Thitivirachawat S, Tanjasiri C, Tungpradabkul S, Hongeng S. Proteomic analysis of β-Thalassemia/HbE: a perspective from hematopoietic stem cells (HSCs). Proteomics technologies and applications: IntechOpen; 2019.
Meisel M, Hinterleitner R, Pacis A, Chen L, Earley ZM, Mayassi T, et al. Microbial signals drive pre-leukaemic myeloproliferation in a Tet2-deficient host. Nature.2018;557:580–4.
Acknowledgements
The authors would like to thank David Malloy, David Maley, Connor Booker, Gretchen Wagner, Emma Cummings for zebrafish care and maintenance, and Jennifer Curran for administrative support. We thank Dr. Todd Druley (Washington University, St. Louis) for his critical review of the paper. We would also like to extend our thanks to the Michael Smith Genome Science Centre for help with sequencing.
Funding
VR was funded by the Cancer Research Training Program of the BHCRI, with funds provided by the Terry Fox Research Institute through the Dr. Linnea Veinotte Memorial Graduate Student Award. VR was also funded by the Nova Scotia Health Research Foundation (NSHRF) Scotia Scholar award.
Author information
Authors and Affiliations
Contributions
VR designed and performed experiments and wrote the paper; KC, RW, and SVP designed and performed experiments and edited the paper; ML is a clinical hematopathologist and performed the hematopathology analysis. MM and AC performed MeDIP-Seq studies and edited the paper; MH oversaw the MeDIP-Seq experiment and edited the paper; JNB originated the research concept, designed experiments, and edited the paper.
Corresponding author
Ethics declarations
Competing interests
JB is a member of the Scientific Advisory Board of Oxford Immune Algorithmics.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Rajan, V., Collett, K., Woodside, R. et al. Stress hematopoiesis induces a proliferative advantage in TET2 deficiency. Leukemia 36, 809–820 (2022). https://doi.org/10.1038/s41375-021-01427-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01427-7
This article is cited by
-
Zebrafish: a convenient tool for myelopoiesis research
Cell Regeneration (2023)